Abstract
Context
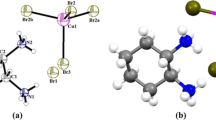
The crystal and molecular structure, electronic properties, optical parameters, and elastic properties of a 1:2 hexanitrohexaazaisowurtzitane (CL-20)/2-mercapto-1-methylimidazole (MMI) cocrystal under 0 ~ 100 GPa hydrostatic pressure were calculated. The results show that the cocrystal CL-20/MMI undergoes three structural transitions at 72 GPa, 95 GPa, and 97 GPa, respectively, and the structural transition occurs in the part of the MMI compound. Structural mutations formed new bonds S1-S2, C2-C7, and N1C5 at 72GPa, 95 GPa, and 97 GPa, respectively. Similarly, the formation of new bonds is confirmed on the basis of an analysis of the changes in lattice constants, cell volumes, and partial densities of states (PDOS) for S1, S2, C2, C7, N1, and C3 at the corresponding pressures. The optical parameters show that the pressure makes the peaks of various optical parameters of CL-20/MMI larger, and the optical activity is enhanced. The optical parameters also confirm the structural mutation of CL-20/MMI under the corresponding pressure.
Method
CL-20/MMI was calculated by using the first-principles norm-conservative pseudopotential based on density functional theory (DFT) in the CASTEP software package. For the optimization results, the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method is selected to optimize the geometry of the cocrystal in the range of 0–100 GPa. GGA/PBE (Perdew–Burke–Ernzerhof) was selected to relax the cocrystal CL-20/MMI fully without constraints at atmospheric pressure. The sampling scheme in the Brillouin zone [10] is the Monkhorst–Pack scheme, and the number of k-point grids was 2 × 2 × 2. By contrast, this study will use the LDA method to calculate.










Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Liu G, Li H, Gou R et al (2018) Packing structures of CL-20-based cocrystals[J]. Cryst Growth Des 18(11):7065–7078
Liu YJ, Lv P, Sun C et al (2019) Syntheses, crystal structures, and properties of two novel CL-20-based cocrystals[J]. Z Anorg Allg Chem 645(8):656–662
Zhu S, Gan Q, Cheng N et al (2020) Exploring the effects of solvents on an organic explosive: insights from the electron structure, electrostatic potential, and conformational transformations of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane[J]. Int J Quantum Chem 120(12):e26202
Hai-jian LI, Chao C, Zhe Z et al (2022) Preparation and thermal decomposition of CL-20-based cocrystals using resonant acoustic mixing[J]. Chinese J Explos Propellants 45(6):848–855. https://doi.org/10.14077/j.issn.1007-7812.202209001
Xue ZH, Huang B, Li H et al (2020) Nitramine-based energetic cocrystals with improved stability and controlled reactivity[J]. Cryst Growth Des 20(12):8124–8147
Ma P, Pan Y, Jiang J et al (2018) Molecular dynamic simulation and density functional theory insight into the nitrogen rich explosive 1, 5-diaminotetrazole (DAT)[J]. Procedia England 211:546–554
Sultan M, Wu J, Haq IU et al (2022) Recent progress on synthesis, characterization, and performance of energetic cocrystals: a review[J]. Molecules 27(15):4775
Clark S J, Segall M D, Pickard CJ et al (2005) First principles methods using CASTEP[J]. Zeitschrift für kristallographie-crystalline Materials, 220(5–6):567–570
Segall MD, Lindan PJD, Probert MJ et al (2002) First-principles simulation: ideas, illustrations and the CASTEP code[J]. Phys 14(11):2717–2744
Chadi DJ (1977) Special points for Brillouin-zone integrations[J]. Phys Rev B 16(4):1746
Sheldrick GM (2015) Crystal structure refinement with SHELXL[J]. Acta Crystallogr Section C: Struct Chem 71(1):3–8
Xiang D, Ji G, Zhu W (2019) Structural and vibrational properties of crystalline b-octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine at high temperatures: ab initio molecular dynamics studies[J]. Chemistry 4(14):4244–4250
Zhu W, Zhang X, Zhu W et al (2008) Density functional theory studies of hydro-static compression of crystalline ammonium perchlorate[J]. Phys Chem Chem Phys 10(48):7318–7323
Fuchs F, Furthmüller J, Bechstedt F, Shishkin M, Kresse G (2007) Quasiparticle band structure based on a generalized Kohn-Sham scheme[J]. Phys Rev B 76(11):115109
Lejaeghere K, Van Speybroeck V, Van Oost G, Cottenier S (2014) Error estimates for solid-state density-functional theory predictions: an overview by means of the ground-state elemental crystals[J]. Crit Rev Solid State Mater Sci 39(1):1–24
Yalameha S, Nourbakhsh Z (2020) Coexistence of type-I and critical-type nodal line states in intermetallic compounds ScM (M=Cu, Ag, Au)[J]. J Phys: Condens Matter 32(29):295502
Zhong M, Qin H, Liu Q J et al (2019) Structures, elasticity, and sensitivity characteristics of ɛ‐CL‐20 under high pressure from first‐principles calculations[J]. Physica Status Solidi (b), 256(5):1800440
Budzevich MM, Landerville AC, Conroy MW et al (2010) Hydrostatic and uniaxial compression studies of 1, 3, 5-triamino-2, 4, 6-trinitrobenzene using density functional theory with van der Waals correction[J]. J Appl Phys 107(11):113524
Zhu WH, Xiao JJ, Ji GF, Zhao F, Xiao HM (2007) First-principles study of the four polymorphs of crystalline octahydro-1,3,5,7-tetranitro- 1,3,5,7-tetrazocine. J Phys Chem B 111:12715–12722
Kuklja MM, Stefanovich EV, Kunz AB (2000) An excitonic mechanism of detonation initiation in explosives[J]. Chem Phys 112:3417–3423
Chen J et al (2023) Molecular design and theoretical study of oxadiazole-bifurazan derivatives[J]. J Mol Model 29(6):1–14
Zhu W, Xiao J, Ji G et al (2007) First-principles study of the four polymorphs of crystalline octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine[J]. J Phys Chem B 111(44):12715–12722
Zhu W, Xiao H (2010) First-principles band gap criterion for impact sensitivity of energetic crystals: a review[J]. Struct Chem 21:657–665
Xu XJ, Zhu WH, Xiao HM (2007) DFT studies on the four polymorphs of crystalline CL-20 and the influences of hydrostatic pressure on ε-CL-20 crystal[J]. J Phys Chem B 111(8):2090–2097
Maurya V, Sharma G, Joshi KB (2021) First-principles characterisation of structural and electronic properties of some RuO2 crystals[J]. Phys Scr 96(5):055807
Liu QJ, Liu ZT, Feng LP et al (2010) First-principles study of structural, elastic, electronic and optical properties of rutile GeO2 and α-quartz GeO2[J]. Solid State Sci 12(10):1748–1755
Saha S, Sinha TP, Mookerjee A (2010) Electronic structure, chemical bonding, and optical properties of paraelectric BaTiO 3[J]. Phys Rev B 62(13):8828
Hayatullah MG, Muhammad S et al (2013) Physical properties of CsSnM_3 (M= Cl, Br, I): a first principle study[J]. Acta Phys Pol, A 124(1):102–107
Subhan F, Azam S, Khan G et al (2019) Elastic and optoelectronic properties of CaTa2O6 compounds: cubic and orthorhombic phases[J]. J Alloy Compd 785:232–239
Ali MA, Hossain MM, Islam A et al (2021) Ternary boride Hf3PB4: insights into the physical properties of the hardest possible boride MAX phase[J]. J Alloy Compd 857:158264
Mouhat F, Coudert FX (2014) Necessary and sufficient elastic stability conditions in various crystal systems[J]. Phys Rev B 90(22):224104
Hadi MA, Monira U, Chroneos A et al (2019) Phase stability and physical properties of (Zr1-xNbx) 2AlC MAX phases[J]. J Phys Chem Solids 132:38–47
Day GM, Price SL, Leslie M (2001) Elastic constant calculations for molecular organic crystals[J]. Cryst Growth Des 1(1):13–27
Hill R (1952) The elastic behaviour of a crystalline aggregate[J]. Proceedings of the Physical Society. Section A, 65(5):349
Pugh SFXCII (1954) Relations between the elastic moduli and the plastic properties of polycrystalline pure metals[J]. London, Edinburgh, Dublin Philos Mag J Sci 45(367):823–843
Seddik T, Semari F, Khenata R et al (2010) High pressure phase transition and elastic properties of Lutetium chalcogenide[J]. Physica B 405(1):394–399
Pettifor DG (1992) Theoretical predictions of structure and related properties of intermetallics[J]. Mater Sci Technol 8(4):345–349
Finnis MW, Sinclair JE (1984) A simple empirical N-body potential for transition metals[J]. Philos Mag A 50(1):45–55
Naher MI, Naqib SH (2020) Structural, elastic, electronic, bonding, and optical properties of topological CaSn3 semimetal[J]. J Alloy Compd 829:154509
Xu J et al (2023) Theoretical study into effects of different substituents on the structure and properties of Keto-RDX compounds[J]. Comput Theor Chem 1224:114111
Mahamudujjaman M, Afzal M A, Islam RS et al (2022) First-principles insights into mechanical, optoelectronic, and thermo-physical properties of transition metal dichalcogenides ZrX2 (X= S, Se, and Te)[J]. AIP Adv 12:025011. https://doi.org/10.1063/5.0073631
Xiao T et al (2023) Theoretical insight into different energetic groups on the performance of energetic materials 2, 5, 7, 9-tetranitro-2, 5, 7, 9-tetraazabicyclo [4, 3, 0] nonane-8-one[J]. J Mol Model 29(8):231
Zhao M et al (2022) Structural transformation of methyl urotropine perchlorate under high pressure[J]. J Mol Model 28(9):251–251
Acknowledgements
We are so grateful to the High-Performance Computing Center of Nanjing Tech University for doing the numerical calculations in this paper on its x-Flex enterprise blade cluster system.
Funding
This study was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Conceptualization: Peng Ma and Jiani Xu; methodology: Jiani Xu; formal analysis and investigation: Jiani Xu, Tingting Xiao, and Jun Chen; writing—original draft preparation: Jiani Xu, Mengjie Bo, and Zhihui Gu; writing—review and editing: Jiani Xu, Peng Ma, and Zikai Gao; funding acquisition: Peng Ma and Congming Ma; resources: Peng Ma and Congming Ma; supervision: Peng Ma and Congming Ma.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Xiao, T., Chen, J. et al. Periodic DFT study of structural transformations of cocrystal CL-20/MMI under high pressure. J Mol Model 30, 124 (2024). https://doi.org/10.1007/s00894-024-05918-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05918-z