Abstract
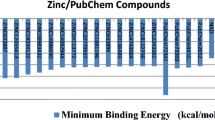
Thymoquinone, as one of the main constituents of black seed, has impressive medicinal properties. In the present study, the interaction of this compound and its five derivatives with phosphatase and tensin homolog (PTEN), which is the second most highly mutated protein in a wide variety of human cancers, has been studied using molecular docking and molecular dynamics simulation studies. Molecular docking results show that thymoquinone derivatives bounded to PTEN and have relatively suitable binding energies. Analysis of molecular dynamics (MD) simulation results suggested that the interactions between thymoquinone analogues and PTEN are stable, and the binding of the ligands limited the flexibility of key residues in the binding site of this protein. Furthermore, data analysis of all the compounds indicates the predominant roles of hydrogen bonds and van der Waals interactions in the ligand binding process to PTEN. Finally, adsorption, distribution, metabolism, and excretion (ADME) analysis predicted that all of these compounds obeyed Lipinski’s RO5 and did not show any violation; therefore, all of these compounds can act like a drug. Results of the current study could shed some light on the binding of thymoquinone analogues to PTEN for further experimental studies.
Graphical abstract








Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Nithya G, Sakthisekaran D (2015) In silico docking studies on the anti-cancer effect of thymoquinone on interaction with phosphatase and tensin homolog located on chromosome 10q23: a regulator of PI3K/AKT pathway. Asian J Pharm Clin Res 8:192–195
Ferlay J, Ferlay J, Shin H-R et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Majdalawieh AF, Fayyad MW (2015) Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol 28:295–304. https://doi.org/10.1016/j.intimp.2015.06.023
Hosseinzadeh H, Eskandari M, Ziaee T (2008) Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs. Pharmacologyonline 2:5
Ulfa SM, Sholikhah S, Utomo EP (2017) Synthesis of thymoquinone derivatives and its activity analysis: in-silico approach. AIP Conf Proc 1823:20102. https://doi.org/10.1063/1.4978175
Gali-Muhtasib H, Roessner A, Schneider-Stock R (2006) Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol 38:1249–1253
Badary OA, Taha RA, Gamal El-Din AM, Abdel-Wahab MH (2003) Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26:87–98. https://doi.org/10.1081/dct-120020404
Nahid Mahmoud Hassan E-A, Manal Mohamed Elhassan T, Siddig Ibrahim A, et al (2015) Anti-diabetic properties of thymoquinone is unassociated with glycogen phosphorylase inhibition. Pharmacogn J. 7
Schneider-Stock R, Fakhoury IH, Zaki AM et al (2014) Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov Today 19:18–30. https://doi.org/10.1016/j.drudis.2013.08.021
Woo CC, Kumar AP, Sethi G, Tan KH (2012) Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol 83:443–451. https://doi.org/10.1016/j.bcp.2011.09.029
Asaduzzaman Khan M, Tania M, Fu S, Fu J (2017) Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget 8:51907–51919. https://doi.org/10.18632/oncotarget.17206
Fujisaki R, Kamei K, Yamamura M et al (2012) In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J Trop Med Public Health 43:270–279
Johnson-Ajinwo OR, Ullah I, Mbye H et al (2018) The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorganic & Medicinal Chemistry Letters 28:1219–1222. https://doi.org/10.1016/j.bmcl.2018.02.051
Antonenko YN, Avetisyan A, v, Bakeeva LE, et al (2008) Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochem Mosc 73:1273–1287. https://doi.org/10.1134/s0006297908120018
Alkharfy KM, Ahmad A, Khan RM, Al-Shagha WM (2015) Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur J Drug Metab Pharmacokinet 40:319–323. https://doi.org/10.1007/s13318-014-0207-8
Effenberger K, Breyer S, Schobert R (2010) Terpene conjugates of the Nigella sativa seed-oil constituent thymoquinone with enhanced efficacy in cancer cells. Chem Biodivers 7:129–139. https://doi.org/10.1002/cbdv.200900328
Breyer S, Effenberger K, Schobert R (2009) Effects of thymoquinone-fatty acid conjugates on cancer cells. ChemMedChem 4:761–768. https://doi.org/10.1002/cmdc.200800430
Effenberger-Neidnicht K, Breyer S, Mahal K et al (2011) Cellular localisation of antitumoral 6-alkyl thymoquinones revealed by an alkyne-azide click reaction and the streptavidin-biotin system. ChemBioChem 12:1237–1241. https://doi.org/10.1002/cbic.201000762
Banerjee S, Azmi AS, Padhye S et al (2010) Structure-activity studies on therapeutic potential of thymoquinone analogs in pancreatic cancer. Pharm Res 27:1146–1158. https://doi.org/10.1007/s11095-010-0145-3
Fröhlich T, Ndreshkjana B, Muenzner JK et al (2017) Synthesis of novel hybrids of thymoquinone and artemisinin with high activity and selectivity against colon cancer. ChemMedChem 12:226–234. https://doi.org/10.1002/cmdc.201600594
Yusufi M, Banerjee S, Mohammad M et al (2013) Synthesis, characterization and anti-tumor activity of novel thymoquinone analogs against pancreatic cancer. Bioorg Med Chem Lett 23:3101–3104. https://doi.org/10.1016/j.bmcl.2013.03.003
Carnero A, Blanco-Aparicio C, Renner O et al (2008) The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 8:187–198. https://doi.org/10.2174/156800908784293659
Dong JT (2006) Prevalent mutations in prostate cancer. J Cell Biochem 97:433–447. https://doi.org/10.1002/jcb.20696
Khan I, Ansari IA, Singh P, Dass JFP (2017) Prediction of functionally significant single nucleotide polymorphisms in PTEN tumor suppressor gene: an in silico approach. Biotechnol Appl Biochem 64:657–666. https://doi.org/10.1002/bab.1483
Guldberg P, thor Straten P, Birck A, et al (1997) Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res 57:3660–3663
Cairns P, Okami K, Halachmi S et al (1997) Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 57:4997–5000
Kohno T, Takahashi M, Manda R, Yokota J (1998) Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosom Cancer 22:152–156. https://doi.org/10.1002/(sici)1098-2264(199806)22:2%3c152::aid-gcc10%3e3.0.co;2-s
Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR (2019) Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin Cancer Biol 59:66–79. https://doi.org/10.1016/j.semcancer.2019.02.001
Zhou Y, Cai S, Moutal A et al (2019) The natural flavonoid naringenin elicits analgesia through inhibition of NaV1.8 voltage-gated sodium channels. ACS Chem Neurosci 10:4834–4846. https://doi.org/10.1021/acschemneuro.9b00547
Bonneau D, Longy M (2000) Mutations of the human PTEN gene. Hum Mutat 16:109–122. https://doi.org/10.1002/1098-1004(200008)16:2%3c109::Aid-humu3%3e3.0.Co;2-0
Lee J-O, Yang H, Georgescu M-M et al (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334
Frisch MJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nak GWT H (2004) Gaussian 03. Gaussian, IncWallingford CT
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chemical Physics 55:117–129. https://doi.org/10.1016/0301-0104(81)85090-2
Mennucci B (2012) Polarizable continuum model. WIREs Comput Mol Sci 2:386–404. https://doi.org/10.1002/wcms.1086
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Morris GM, Goodsell DS, Halliday RS et al (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
E. Lindahl B. Hess, D. van der Spoel. MJA (2020) GROMACS 2019.6. 10.5281/zenodo.3685922
Hermans J, Berendsen HJC, van Gunsteren WF, Postma JPM (1984) A consistent empirical potential for water–protein interactions. Biopolymers 23:1513–1518. https://doi.org/10.1002/bip.360230807
Malde AK, Zuo L, Breeze M et al (2011) An automated force field topology builder (ATB) and repository: version 1.0. J Chem Theory Comput 7:4026–4037. https://doi.org/10.1021/ct200196m
Jorgensen WL, Chandrasekhar J, Madura JD et al (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Evans DJ, Holian BL (1985) The nose–hoover thermostat. J Chem Phys 83:4069–4074
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:14101
Berendsen HJC, van Postma JPM, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅ log (N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Daina A, Zoete V (2016) A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 11:1117–1121. https://doi.org/10.1002/cmdc.201600182
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:1–13
Renug S, Muthu S (2014) Vibrational spectra and normal coordinate analysis of 2-hydroxy-3-(2-methoxyphenoxy) propyl carbamate. Spect Acta A: Mol Bio Spect 132:313–325
Pearson RG (1989) Absolute electronegativity and hardness: applications to organic chemistry. J Org Chem 54:1423–1430
de Ruyck J, Brysbaert G, Blossey R, Lensink MF (2016) Molecular docking as a popular tool in drug design, an in silico travel. Adv Appl Bioinform Chem 9:1–11. https://doi.org/10.2147/aabc.S105289
Muthumanickam S, Indhumathi T, Boomi P, et al (2020) In silico approach of naringin as potent phosphatase and tensin homolog (PTEN) protein agonist against prostate cancer. J Biomol Struct Dyn 1–10
Liu Y, Liu Z, Zeng G et al (2018) Effect of surfactants on the interaction of phenol with laccase: molecular docking and molecular dynamics simulation studies. J Hazard Mater 357:10–18. https://doi.org/10.1016/j.jhazmat.2018.05.042
Chen M, Zeng G, Lai C et al (2017) Interactions of carbon nanotubes and/or graphene with manganese peroxidase during biodegradation of endocrine disruptors and triclosan. Chemosphere 184:127–136. https://doi.org/10.1016/j.chemosphere.2017.05.162
Li X, Zheng Y (2017) Lignin-enzyme interaction: mechanism, mitigation approach, modeling, and research prospects. Biotechnol Adv 35:466–489. https://doi.org/10.1016/j.biotechadv.2017.03.010
Kumar N, Srivastava R, Prakash A, Lynn AM (2020) Structure-based virtual screening, molecular dynamics simulation and MM-PBSA toward identifying the inhibitors for two-component regulatory system protein NarL of Mycobacterium tuberculosis. J Biomol Struct Dyn 38:3396–3410. https://doi.org/10.1080/07391102.2019.1657499
Abdizadeh R, Hadizadeh F, Abdizadeh T (2021) In silico analysis and identification of antiviral coumarin derivatives against 3-chymotrypsin-like main protease of the novel coronavirus SARS-CoV-2. Mol Diversity. https://doi.org/10.1007/s11030-021-10230-6
Reimer PJ, Bard E, Bayliss A et al (2013) IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55:1869–1887
Author information
Authors and Affiliations
Contributions
The authors contributed to the study conception and design. The first draft of the manuscript was written by Hokmabady, and authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hokmabady, L., Fani, N. In silico elucidation of the interactions of thymoquinone analogues with phosphatase and tensin homolog (PTEN). J Mol Model 28, 321 (2022). https://doi.org/10.1007/s00894-022-05318-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05318-1