Abstract
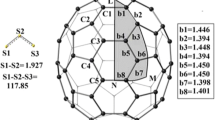
The formation of C70O from C70O3 monomolozonide is a three-step process with the isomer dependent last step leading either to c,c-C70O epoxide or d,d-C70O oxidoannulene. The process involves the open intermediate (first O–O then Cc–Cc/Cd–Cd bonds broken), oxidoannulene-like structure intermediate (new Cc–O/Cd–O bond formed) and finally the oxide product. On the formation of c,c-C70O isomer, the final release of O2 is followed by the restoration of Cc–Cc bond, which stabilizes the product. Neither Cd–Cd bond is restored nor the total energy essentially lowered upon d,d-C70O formation. At all steps of the studied process, the four CC bonds adjacent to Cc–Cc or Cd–Cd bond, respectively, play a crucial role donating or withdrawing the necessary electron density. C70(O)O2 products, with O2 bridging one of the bonds adjacent to the parent Cc–Cc/Cd–Cd one, may compete with the oxide products. The OO bond in such structures is weak as suggested by its low electron population. For both c,c-C70O3 and d,d-C70O3, the shape of the potential energy surfaces (0 K) and the related, reported earlier, room temperature–free energy surfaces differ.

Graphical abstract









Similar content being viewed by others
References
Diederich F, Ettl R, Rubin Y, Whetten RL, Beck R, Alvarez M, Anz S, Sensharma D, Wudl F, Khemani KC, Koch A (1991) The higher fullerenes: isolation and characterization of C76, C84, C90, C94, and C70O, an oxide of D5h-C70. Science 252:548–551. https://doi.org/10.1126/science.252.5005.548
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: buckminsterfullerene. Nature. 318:162–163. https://doi.org/10.1038/318162a0
Heymann D, Bachilo SM, Weisman RB (2002) Ozonides, epoxides, and oxidoannulenes of C70. J Am Chem Soc 124:6317–6323. https://doi.org/10.1021/ja012488p
Heymann D, Weisman RB (2006) Fullerene oxides and ozonides. Comptes Rendus Chim 9:1107–1116. https://doi.org/10.1016/J.CRCI.2006.02.003
Bil A, Latajka Z, Morrison CA (2009) C70 oxides and ozonides and the mechanism of ozonolysis on the fullerene surface. A theoretical study. J Phys Chem A 113:9891–9898. https://doi.org/10.1021/jp9024798
Bil A, Latajka Z, Hutter J, Morrison CA (2014) Describing the chemical bonding in C70 and C70O3 – a quantum chemical topology study. Chem Phys 433:22–30. https://doi.org/10.1016/j.chemphys.2014.02.003
Bil A, Latajka Z, Morrison CA (2014) Density functional theory based molecular dynamics simulations of C70O3 doped with light molecules. Chem Phys 428:121–126. https://doi.org/10.1016/j.chemphys.2013.10.011
Bil A, Morrison CA (2012) Modifying the fullerene surface using endohedral noble gas atoms: density functional theory based molecular dynamics study of C70O3. J Phys Chem A 116:3413–3419. https://doi.org/10.1021/jp210529y
Bil A, Hutter J, Morrison CA (2014) Electron transfer modifies chemical properties of C70 fullerene surface: an ab initio molecular dynamics study of C70O3 molozonides doped with light atoms. Chem Phys Lett 605–606:93–97. https://doi.org/10.1016/j.cplett.2014.05.025
Bil A (2019) The mechanism of ozonolysis on the surface of C70 fullerene. The free energy surface theoretical study. J Mol Struct 1185:361–368. https://doi.org/10.1016/J.MOLSTRUC.2019.03.005
Huang Y-S, Wang G-W (2008) Ozonization of C70 and subsequent thermolysis of ozonide 1,2-C70O3: a theoretical study. J Mol Struct THEOCHEM 860:24–31. https://doi.org/10.1016/J.THEOCHEM.2008.03.010
Criegee R (1975) Mechanism of Ozonolysis. Angew Chem Int Ed Eng 14:745–752. https://doi.org/10.1002/anie.197507451
Geletneky C, Berger S (1998) The mechanism of ozonolysis revisited by 17O-NMR spectroscopy. Eur J Org Chem 1998:1625–1627. https://doi.org/10.1002/(SICI)1099-0690(199808)1998:8<1625::AID-EJOC1625>3.0.CO;2-L
Henkelman G, Jónsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys 113:9978–9985. https://doi.org/10.1063/1.1323224
Kohout M (2004) A measure of electron localizability. Int J Quantum Chem 97:651–658. https://doi.org/10.1002/qua.10768
Kohout M, Pernal K, Wagner FR, Grin Y (2004) Electron localizability indicator for correlated wavefunctions. I. Parallel-spin pairs. Theor Chem Accounts 112:453–459. https://doi.org/10.1007/s00214-004-0615-y
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403. https://doi.org/10.1063/1.458517
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature. 371:683–686. https://doi.org/10.1038/371683a0
Kohout M (2007) Bonding indicators from electron pair density functionals. Faraday Discuss 135:43–54. https://doi.org/10.1039/B605951C
Bil A, Latajka Z (2004) Examination of the hydroperoxy radical and its closed-shell “analogues” – the protonation sites: topological predictions and ab initio study of the protonated forms. Chem Phys 305:243–252. https://doi.org/10.1016/j.chemphys.2004.06.062
Bil A, Latajka Z (2004) The examination of the hydroperoxy radical and its closed-shell “analogues” by means of topological methods of quantum chemistry: AIM and ELF. Chem Phys 303:43–53. https://doi.org/10.1016/j.chemphys.2004.05.020
Knapp CE, Wann DA, Bil A, Schirlin JT, Robertson HE, McMillan PF, Rankin DWH, Carmalt CJ (2012) Dimethylalkoxygallanes: monomeric versus dimeric gas-phase structures. Inorg Chem 51:3324–3331. https://doi.org/10.1021/ic202775x
Bil A, Grzechnik K, Mierzwicki K, Mielke Z (2013) OH-induced oxidative cleavage of dimethyl disulfide in the presence of NO. J Phys Chem A 117:8263–8273. https://doi.org/10.1021/jp4047837
Andrés J, Berski S, Silvi B (2016) Curly arrows meet electron density transfers in chemical reaction mechanisms: from electron localization function (ELF) analysis to valence-shell electron-pair repulsion (VSEPR) inspired interpretation. Chem Commun 52:8183–8195. https://doi.org/10.1039/C5CC09816E
Krokidis X, Goncalves V, Savin A, Silvi B (1998) How malonaldehyde bonds change during proton transfer. J Phys Chem A 102:5065–5073. https://doi.org/10.1021/jp9734282
Bil A, Latajka Z, Biczysko M (2018) Hydrogen detachment driven by a repulsive 1πσ* state – an electron localization function study of 3-amino-1,2,4-triazole. Phys Chem Chem Phys 20:5210–5216. https://doi.org/10.1039/C7CP06744E
Krokidis X, Silvi B, Dezarnaud-Dandine C, Sevin A (1998) Topological study, using a coupled ELF and catastrophe theory technique, of electron transfer in the Li+Cl2 system. New J Chem 22:1341. https://doi.org/10.1039/a801838c
Becke A (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100 http://pra.aps.org/abstract/PRA/v38/i6/p3098_1. Accessed 17 Mar 2014
Lee C, Yang W, Parr R (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37 http://prb.aps.org/abstract/PRB/v37/i2/p785_1. Accessed 17 Mar 2014
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Lippert G, Hutter J, Parrinello M (1997) A hybrid Gaussian and plane wave density functional scheme. Mol Phys 92:477–487
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput Phys Commun 167:103–128. https://doi.org/10.1016/j.cpc.2004.12.014
The CP2K developers group http://cp2k.org/, CP2K
Goedecker S, Teter M (1996) Separable dual-space Gaussian pseudopotentials. Phys Rev B - Condens Matter Mater Phys 54:1703–1710. https://doi.org/10.1103/PhysRevB.54.1703
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100:5829–5835. https://doi.org/10.1063/1.467146
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09
Kohout M (2011) DGrid-4.6
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Llusar R, Beltran A, Andres J, Noury S, Silvi B (1999) Topological analysis of electron density in depleted homopolar chemical bonds. J Comput Chem 20:1517–1526. https://doi.org/10.1002/(SICI)1096-987X(19991115)20:14<1517::AID-JCC4>3.0.CO;2-#
Berski S, Mierzwicki K, Bil A, Latajka Z (2008) The protocovalent NO bond: quantum chemical topology (QCT of ELF and ELI-D) study on the bonding in the nitrous acid HONO and its relevancy to the experiment. Chem Phys Lett 460:559–562. https://doi.org/10.1016/j.cplett.2008.06.051
Chapleski RC, Morris JR, Troya D (2014) A theoretical study of the ozonolysis of C60: primary ozonide formation, dissociation, and multiple ozone additions. Phys Chem Chem Phys 16:5977–5986. https://doi.org/10.1039/c3cp55212h
Xin N, Yang X, Zhou Z, Zhang J, Zhang S, Gan L (2013) Synthesis of C60 (O)3 : an open-cage fullerene with a ketolactone moiety on the orifice. J Org Chem 78:1157–1162. https://doi.org/10.1021/jo3026302
Kudo T, Akimoto Y, Shinoda K, Jeyadevan B, Tohji K, Nirasawa T, Waelchli M, Krätschmer W (2002) Characterization and structures of dimeric C70 oxides, C140O, synthesized with hydrothermal treatment. J Phys Chem B 106:4383–4389. https://doi.org/10.1021/jp0139989
Acknowledgments
The authors would like to express personal gratitude to professor Zdzisław Latajka, a noble man and a supervisor of their PhD theses, for fruitful long-standing collaboration.
Funding
A grant of computer time from the Wrocław Center for Networking and Supercomputing (WCSS) is gratefully acknowledged. A.B. would like to thank the Ministry of Science and Higher Education, Republic of Poland, for supporting this work under the grant no. N N204 280738.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor Zdzislaw Latajka, on occasion of his 70th birthday in recognition on his vital research contributions.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection Zdzislaw Latajka 70th Birthday Festschrift
Electronic supplementary material
ESM 1
Figs. S1–S20 and Cartesian coordinates of the relevant stationary structures are available as Electronic supplementary material as well as extended discussion and additional comments concerning the bonds evolution along the reaction paths studied in the main body of the paper. (DOCX 5823 kb)
Rights and permissions
About this article
Cite this article
Bil, A., Mierzwicki, K. The mechanism of the ozonolysis on the surface of C70 fullerene: the electron localizability indicator study. J Mol Model 26, 73 (2020). https://doi.org/10.1007/s00894-020-4333-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4333-8