Abstract
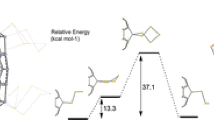
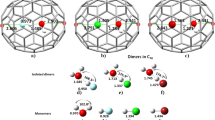
The first results of the hydrogenation of S6-symmetric trifluoromethylfullerene C60(CF3)12 in two types of reactions were reported: (1) high-temperature radical hydrogenation with 9,10-dihydroanthracene and (2) nucleophilic hydrogenation with sodium tetraborohydride under mild conditions. The high-temperature radical hydrogenation of S6-C60(CF3)12 is accompanied by partial elimination of CF3 groups and leads to the formation of a complex mixture of products of a composition C60(CF3)8–12H18–22. During the hydrogenation of NaBH4 under mild conditions, selective formation of the hydride C60(CF3)12H12 was recorded by mass spectroscopy. A kinetic analysis of the sequential nucleophilic hydrogenation of S6-C60(CF3)12 was performed, using quantum-chemical modeling at the level of density functional theory, under the assumption of linear correlation between the activation energy and the enthalpy of elementary steps of the same type. The isomeric composition was predicted for the series of anionic intermediates C60(CF3)12H\(_{{_{{2n-1}}}}^{ - }\) and their protonation products C60(CF3)12H2n, where n = 1–6. The hydrogenation of S6-C60(CF3)12 should lead to the formation of the thermodynamically and kinetically most stable product ortho-S6-C60(CF3)12H12, in which all hydrogen atoms are located in neighboring positions near the CF3 groups, forming together with them a near-equatorial belt of 24 addends while retaining the triphenylene fragments at two opposite poles. The average bond dissociation energy BDE(C–H) in ortho-S6-C60(CF3)12H12 is 298 kJ mol–1, which is approximately 20 kJ mol–1 higher than the BDE(C–H) of known fullerene hydrides C60H18 and C60H36 (PBE0/def2-SVP).









Similar content being viewed by others
REFERENCES
N. F. Gol’dshleger and A. P. Moravskii, Russ. Chem. Rev. 66, 323 (1997).
J. Nossal, R. K. Saini, L. B. Alemany, et al., Eur. J. Org. Chem., 4167 (2001).
R. Taylor, J. Fluorine Chem. 125, 359 (2004).
A. A. Goryunkov, N. S. Ovchinnikova, I. V. Trushkov, et al., Russ. Chem. Rev. 76, 289 (2007).
S. I. Troyanov and E. Kemnitz, Curr. Org. Chem. 16, 1060 (2012).
S. I. Troyanov, A. Dimitrov, and E. Kemnitz, Angew. Chem., Int. Ed. Engl. 45, 1971 (2006).
S. I. Troyanov and E. Kemnitz, Mendeleev Commun. 18, 27 (2008).
P. A. Berseth, A. G. Harter, R. Zidan, et al., Nano Lett. 9, 1501 (2009).
R. H. Scheicher, S. Li, C. M. Araujo, et al., Nanotecnology 22, 335401 (2011).
V. A. Brotsman, N. S. Lukonina, and A. A. Goryunkov, Russ. Chem. Bull. 72, 20 (2023).
A. V. Rybalchenko, T. V. Magdesieva, V. A. Brotsman, et al., Electrochim. Acta 174, 143 (2015).
V. P. Bogdanov, O. O. Semivrazhskaya, N. M. Belov, et al., Chem.-Eur. J. 22, 15485 (2016).
V. A. Brotsman, V. P. Bogdanov, A. V. Rybalchenko, et al., Chem. Asian J. 11, 1945 (2016).
W. H. Powell, F. Cozzi, G. P. Moss, et al., Pure Appl. Chem. 74, 629 (2002).
Y. Y. Duan, L. Shi, L. Q. Sun, et al., Int. J. Thermophys. 21, 393 (2000).
L. Banfi, E. Narisano, R. Riva, et al., in Encyclopedia of Reagents for Organic Synthesis (Wiley, Chichester, UK, 2014), p. 1.
J. A. Rackers, Z. Wang, C. Lu, et al., J. Chem. Theory Comput. 14, 5273 (2018).
A. A. Granovsky, Firefly v. 8.2.0 (Formerly PC GAMESS)2016. http://classic.chem.msu.su/gran/firefly/index.html.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem. 14, 1347 (1993).
D. N. Laikov, Chem. Phys. Lett. 281, 151 (1997).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996).
C. Adamo and V. Barone, J. Chem. Phys. 110, 6158 (1999).
F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys. 7, 3297 (2005).
D. V. Ignat’eva, A. A. Goryunkov, I. N. Ioffe, et al., J. Phys. Chem. A 117, 13009 (2013).
N. A. Romanova, T. S. Papina, V. A. Luk’yanova, et al., J. Chem. Thermodyn. 66, 59 (2013).
F. N. Tebbe, R. L. Harlow, D. B. Chase, et al., Science (Washington, DC, U. S.) 256, 822 (1992).
S. M. Pimenova, S. V. Melkhanova, V. P. Kolesov, et al., J. Phys. Chem. B 106, 2127 (2002).
T. Papina, V. Luk’yanova, S. Troyanov, N. V. Chelovskaya, A. G. Buyanovskaya, and L. N. Sidorov, Russ. J. Phys. Chem. A 81, 159 (2007).
C. Ruchardt, M. Gerst, J. Ebenhoch, et al., Angew. Chem., Int. Ed. Engl. 32, 584 (1993).
A. D. Darwish, A. G. Avent, R. Taylor, et al., J. Chem. Soc., Perkin Trans. 2, 2051 (1996).
A. A. Gakh, A. Y. Romanovich, and A. Bax, J. Am. Chem. Soc. 125, 7902 (2003).
C. Rüchardt, M. Gerst, and M. Nölke, Angew. Chem., Int. Ed. Engl. 31, 1523 (1992).
G.-W. Wang, Y.-J. Li, F.-B. Li, et al., Lett. Org. Chem. 2, 595 (2005).
V. Y. Markov, A. Y. Borschevsky, and L. N. Sidorov, Int. J. Mass Spectrom. Ion Process. 325–327, 100 (2012).
R. V. Khatymov, V. Y. Markov, R. F. Tuktarov, et al., Int. J. Mass Spectrom. Ion Process. 272, 119 (2008).
R. V. Khatymov, R. F. Tuktarov, V. Y. Markov, et al., JETP Lett. 96, 659 (2013).
N. A. Romanova, N. B. Tamm, V. Y. Markov, et al., Mendeleev Commun. 22, 297 (2012).
M. P. Kosaya, T. S. Yankova, A. V. Rybalchenko, et al., J. Phys. Chem. A 125, 7876 (2021).
O. N. Lavrent’eva and N. A. Romanova, in Abstracts of Invited Lectures and Contributed Papers (Russia, St. Petersburg, 2015), p. 77.
H. Çelikkan, M. Şahin, M. L. Aksu, et al., Int. J. Hydrogen Energy 32, 588 (2007).
H. P. Spielmann, G.-W. Wang, M. S. Meier, et al., Org. Chem. 63, 9865 (1998).
H. P. Spielmann, B. R. Weedon, and M. S. Meier, Org. Chem. 65, 2755 (2000).
B. W. Clare and D. L. Kepert, J. Mol. Struct.: THEOCHEM 315, 71 (1994).
B. W. Clare and D. L. Kepert, J. Mol. Struct.: THEOCHEM 621, 211 (2003).
B. W. Clare and D. L. Kepert, J. Mol. Struct.: THEOCHEM 622, 185 (2003).
D. L. Kepert and B. W. Clare, Coord. Chem. Rev. 155, 1 (1996).
B. W. Clare and D. L. Kepert, J. Mol. Struct.: THEOCHEM 303, 1 (1994).
O. V. Boltalina, V. Y. Markov, R. Taylor, et al., Chem. Commun. 22, 2549 (1996).
B. W. Clare and D. L. Kepert, J. Mol. Struct. 536, 99 (2001).
O. V. Boltalina, V. Y. Markov, P. A. Troshin, et al., Angew. Chem., Int. Ed. Engl. 40, 787 (2001).
A. A. Popov, A. A. Goryunkov, I. V. Goldt, et al., J. Phys. Chem. A 108, 11449 (2004).
N. B. Shustova, Z. Mazej, Y.-S. Chen, et al., Angew. Chem. Int. J 49, 812 (2010).
O. V. Boltalina, A. A. Goryunkov, V. Y. Markov, et al., Int. J. Mass Spectrom. Ion Process. 228, 807 (2003).
G. V. Lier, M. Cases, C. P. Ewels, et al., J. Org. Chem. 70, 1565 (2005).
C. P. Ewels, G. van Lier, P. Geerlings, et al., J. Chem. Inf. Model. 47, 2208 (2007).
E. I. Dorozhkin, D. V. Ignat’eva, N. B. Tamm, et al., Chem.-Eur. J. 12, 3876 (2006).
E. I. Dorozhkin, A. A. Goryunkov, I. N. Ioffe, et al., Eur. J. Org. Chem., 5082 (2007).
N. M. Belov, M. G. Apenova, A. V. Rybalchenko, et al., Chem.-Eur. J. 20, 1126 (2014).
N. B. Tamm, M. A. Fritz, N. A. Romanova, et al., Chem. Sel. 7 (44) (2022). https://doi.org/10.1002/slct.202202214
N. B. Tamm, M. A. Fritz, N. A. Romanova, et al., Chem. Sel. 7 (19) (2022). https://doi.org/10.1002/slct.202200968
N. A. Romanova, M. A. Fritts, K. Chang, N. B. Tamm, A. A. Goryunkov, L. N. Sidorov, K. Scheurell, E. Kemnitz, and S. I. Troyanov, Russ. Chem. Bull. 63, 2657 (2014).
N. A. Romanova, M. A. Fritz, K. Chang, et al., Chem.-Eur. J. 19, 11707 (2013).
S. I. Troyanov, A. A. Goryunkov, E. I. Dorozhkin, et al., J. Fluorine Chem. 128, 545 (2007).
N. A. Samokhvalova, P. A. Khavrel, V. Y. Markov, et al., Eur. J. Org. Chem., 2935 (2009).
M. P. Kosaya, A. V. Rybalchenko, N. S. Lukonina, et al., Chem. Asian J. 13, 1920 (2018).
P. A. Khavrel, M. G. Serov, G. G. Petukhova, et al., J. Fluorine Chem., 109598 (2020).
M. G. Apenova, O. O. Semivrazhskaya, E. V. Borkovskaya, et al., Chem. Asian J. 10, 1370 (2015).
N. S. Ovchinnikova, A. A. Goryunkov, P. A. Khavrel, et al., Dalton Trans. 40, 959 (2011).
D. C. Wigfield, Tetrahedron 35, 449 (1979).
R. G. Bergosh, M. S. Meier, J. A. Laske Cooke, et al., Org. Chem. 62, 7667 (1997).
ACKNOWLEDGMENTS
The authors are grateful to N.M. Belov for recording the NMR spectra.
Funding
N.A.R. acknowledges the support of the Russian Science Foundation, project no. 22-73-10042. This work was performed using the equipment purchased within the framework of the federal project “Development of Infrastructure for Scientific Research and Training” of the national project “Science and Universities.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Kudrinskaya
Supplementary Information
Rights and permissions
About this article
Cite this article
Romanova, N.A., Markov, V.Y. & Goryunkov, A.A. Hydrogenation of S6-C60(CF3)12. Russ. J. Phys. Chem. 97, 1964–1977 (2023). https://doi.org/10.1134/S0036024423090200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423090200