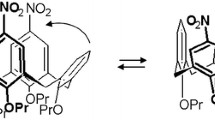
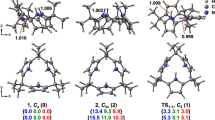
Abstract
Herein, we have investigated the key functions of the calix[4]arene, abbreviated as CX [1], and designed its several derivatives by substitution of the functional groups. Molecular geometry provides an intuitive understanding of the effect of functional groups on various physical properties. The addition of the N-β-ketoimine (n = 1–4) ligands has a direct effect on the stretching vibration of the H-bonding interaction. The results showed that all molecules possess absorption bands at 190 nm and in the range between 200 and 300 nm assigned to π–π* and n-π* transitions. HOMO–LUMO energy gap of the CX[4]-N-β-ketoimine, one with chemical hardness of 1.62 eV, has been found to be 3.24 eV calculated at B3LYP/6–31 + G(d) level of theory. This finding explains the good kinetic stability of this compound. The large values of electrophilicity make the current molecules as a good electrophilic species. The atom in molecule (AIM) and the reduced density gradient (RDG) analyses showed the type and the strength of the interactions taking place between Cu2+ and the β-ketoimine ligands.










Similar content being viewed by others
References
Zhou J, Yu G, Shao L et al (2015) A water-soluble biphen[3]arene: synthesis, host–guest complexation, and application in controllable self-assembly and controlled release. Chem Commun 51:4188–4191. https://doi.org/10.1039/C5CC00225G
Ogoshi T, Kitajima K, Aoki T et al (2010) Synthesis and conformational characteristics of alkyl-substituted pillar[5]arenes. J Organomet Chem 75:3268–3273. https://doi.org/10.1021/jo100273n
Chen L, Wang Y, Wan Y et al (2020) Highly efficient and selective pillararene-based organic materials for Hg2+ and CH3Hg+ extraction from aqueous solution. Chem Eng J 387:124087. https://doi.org/10.1016/j.cej.2020.124087
Panneerselvam M, Kumar MD, Jaccob M, Solomon RV (2018) Computational unravelling of the role of alkyl groups on the host-guest complexation of pillar[5]arenes with neutral dihalobutanes. ChemistrySelect 3:1321–1334. https://doi.org/10.1002/slct.201702541
Shinkai S (1993) Calixarenes - the third generation of supramolecules. Tetrahedron 49:8933–8968. https://doi.org/10.1016/S0040-4020(01)91215-3
Gutsche CD (1989) In: Stoddart JF (ed) Calixarenes, Monographs in Supramolecular Chemistry. the Royal Society of Chemistry, Cambridge
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107:3780–3799. https://doi.org/10.1021/cr068046j
Balasaheb Nimse S, Kim T (2013) Biological applications of functionalized calixarenes. Chem Soc Rev 42:366–386. https://doi.org/10.1039/C2CS35233H
McIldowie MJ, Mocerino M, Ogden MI (2010) A brief review of C n -symmetric calixarenes and resorcinarenes. Supramol Chem 22:13–39. https://doi.org/10.1080/10610270902980663
Vicens J, Böhmer V (2012) Calixarenes: a versatile class of macrocyclic compounds. Springer Science & Business Media, Berlin, p 3
Dondoni A, Marra A (2010) Calixarene and calixresorcarene glycosides: their synthesis and biological applications. Chem Rev 110:4949–4977. https://doi.org/10.1021/cr100027b
Lande DN, Bhadane SA, Gejji SP (2017) Noncovalent interactions accompanying encapsulation of resorcinol within azacalix[4]pyridine macrocycle. J Phys Chem A 121:1814–1824. https://doi.org/10.1021/acs.jpca.6b12912
Wang M-X (2008) Heterocalixaromatics, new generation macrocyclic host molecules in supramolecular chemistry. Chem Commun 38:4541–4551. https://doi.org/10.1039/B809287G
de Ramírez FM, Serrano-Valero E, Varbanov S (2020) Octaphosphinoylated para-tert-butylcalix[8]arene as an extracting agent for uranyl ions in an acidic nitrate medium: study of the extracted uranyl calixarene compound. J Radioanal Nucl Chem 323:651–662. https://doi.org/10.1007/s10967-019-06969-w
Sayin S, Ozyilmaz E, Oguz M et al (2020) Calixarenes functionalised water-soluble iron oxide magnetite nanoparticles for enzyme immobilisation. Supramol Chem 32:1–11. https://doi.org/10.1080/10610278.2020.1740704
Gassoumi B, Ghalla H, Chaabane RB (2020) Host-guest complexation studies of NO3, NO2, CO2, and N2 gas with the calix[4]arene molecule. J Mol Model 26:149. https://doi.org/10.1007/s00894-020-04416-2
Gassoumi B, Ghalla H, Chaabane RB (2019) DFT and TD-DFT investigation of calix[4]arene interactions with TFSI− ion. Heliyon 5:e02822. https://doi.org/10.1016/j.heliyon.2019.e02822
Onac C, Kaya A, Ataman D et al (2018) The removal of Cr(VI) through polymeric supported liquid membrane by using calix[4]arene as a carrier. Chin J Chem Eng 27:85–91. https://doi.org/10.1016/j.cjche.2018.01.029
Cay S, Sayin S, Engin MS (2020) Calix[4]arene embedded polyamide supported liquid membrane for separation of heavy metals from aqueous solutions. J Agric Food Sci Tech 8:387–391. https://doi.org/10.24925/turjaf.v8i2.387-391.3064
Aoki Y, Hirai N, Sakaguchi S, Ishii Y (2005) Aerobic oxidation of 1,3,5-triisopropylbenzene using N-hydroxyphthalimide (NHPI) as key catalyst. Tetrahedron 61:10995–10999. https://doi.org/10.1016/j.tet.2005.08.087
Temel F (2020) One novel calix[4]arene based QCM sensor for sensitive, selective and high performance-sensing of formaldehyde at room temperature. Talanta 211:120725. https://doi.org/10.1016/j.talanta.2020.120725
Prata JV, Costa AI, Teixeira CM (2020) A solid-state fluorescence sensor for nitroaromatics and nitroanilines based on a conjugated calix[4]arene polymer. J Fluoresc 30:41–50. https://doi.org/10.1007/s10895-019-02466-1
Phichi M, Imyim A, Tuntulani T, Aeungmaitrepirom W (2020) Paper-based cation-selective optode sensor containing benzothiazole calix[4]arene for dual colorimetric Ag+ and Hg2+ detection. Anal Chim Acta 1104:147–155. https://doi.org/10.1016/j.aca.2020.01.005
Erdemir S, Malkondu S (2020) Calix[4]arene based a NIR-fluorescent sensor with an enhanced stokes shift for the real-time visualization of Zn(II) in living cells. Sensors Actuators B Chem 306:127574. https://doi.org/10.1016/j.snb.2019.127574
Eddaif L, Shaban A, Telegdi J, Szendro I (2020) A piezogravimetric sensor platform for sensitive detection of lead (II) ions in water based on calix[4]resorcinarene macrocycles: synthesis, characterization and detection. Arab J Chem 13:4448–4461. https://doi.org/10.1016/j.arabjc.2019.09.002
Bhatt M, Paul P (2018) Copper(II)-induced gel formation of a carboxylate functionalized calix[4]arene and its application for sensing of iodide. Soft Materials 16:62–70. https://doi.org/10.1080/1539445X.2017.1406859
Colom E, Andrés-Castán JM, Barrios D et al (2019) Modification of the electronic properties of the π-spacer of chromophores linked to calix[4]arene platform for DSSCs applications. Dyes Pigments 164:43–53. https://doi.org/10.1016/j.dyepig.2018.12.066
Najlaoui D, Echabaane M, Rouis A, Bonnamour I (2019) Morphological, electrical and dielectric characteristics of chromogenic calix[4]arene amide/SWCNT nanocomposite films for device applications. Appl Phys A Mater Sci Process 125:355. https://doi.org/10.1007/s00339-019-2657-y
Sharma VS, Shah AP, Sharma AS, Athar M (2019) Columnar self-assembly, gelation and electrochemical behavior of cone-shaped luminescent supramolecular calix[4]arene LCs based on oxadiazole and thiadiazole derivatives. New J Chem 43:1910–1925. https://doi.org/10.1039/C8NJ04922J
Akpinar M, Temel F, Tabakci B et al (2019) A phenyl glycinol appended calix[4]arene film for chiral detection of ascorbic acid on gold surface. Anal Biochem 583:113373. https://doi.org/10.1016/j.ab.2019.113373
Cao D, Ren F, Feng Y et al (2010) A B3LYP and MP2 theoretical investigation into host-guest interaction between calix[4]arene and Li+ or Na+. J Mol Model 16:589–598. https://doi.org/10.1007/s00894-009-0573-3
Begel S, Puchta R, van Eldik R (2014) Host-guest complexes of calix[4]tubes - prediction of ion selectivity by quantum chemical calculations VI. J Mol Model 20:2200. https://doi.org/10.1007/s00894-014-2200-1
Gong H, Ren F, Zhao L et al (2020) Hydration and swelling: a theoretical investigation on the cooperativity effect of H-bonding interactions between p-hydroxy hydroxymethyl calix[4]/[5]arene and H2O by many-body interaction and density functional reactivity theory. J Mol Model 26:190. https://doi.org/10.1007/s00894-020-04442-0
Lee MH, Quang DT, Jung HS et al (2007) Ion-induced FRET on−off in fluorescent calix[4]arene. J Organomet Chem 72:4242–4245. https://doi.org/10.1021/jo070361y
Ikeda A, Shinkai S (1994) On the origin of high ionophoricity of 1,3-alternate calix[4]arenes: .pi.-donor participation in complexation of cations and evidence for metal-tunneling through the calix[4]arene cavity. J Am Chem Soc 116:3102–3110. https://doi.org/10.1021/ja00086a045
Baur M, Frank M, Schatz J, Schildbach F (2001) Water-soluble calix[n]arenes as receptor molecules for non-polar substrates and inverse phase transfer catalysts. Tetrahedron 57:6985–6991. https://doi.org/10.1016/S0040-4020(01)00653-6
D’Alessandro F, Gulino FG, Impellizzeri G et al (1994) A new water soluble host compound possessing two different hydrophobic recognition cavities: calix[4]arene derivative conjugated with monofunctionalized β-cyclodextrin. Tetrahedron Lett 35:629–632. https://doi.org/10.1016/S0040-4039(00)75856-4
Dalgarno SJ, Atwood JL, Raston CL (2006) Sulfonatocalixarenes: molecular capsule and ‘Russian doll’ arrays to structures mimicking viral geometry. Chem Commun 44:4567–4574. https://doi.org/10.1039/B606803K
Saponar A, Popovici E-J, Perhaita I et al (2012) Thermal behaviour of some ester derivatives of p-tert-butyl calix[n]arene. J Therm Anal Calorim 110:349–356. https://doi.org/10.1007/s10973-012-2415-2
Perret F, Lazar AN, Coleman AW (2006) Biochemistry of the para -sulfonato-calix[n]arenes. Chem Commun 51:2425–2438. https://doi.org/10.1039/B600720C
Sun Z, Zhao Y, Santoro O et al (2020) Use of titanocalix[4]arenes in the ring opening polymerization of cyclic esters. Catal Sci Technol 10:1619–1639. https://doi.org/10.1039/C9CY02571E
Tlustý M, Eigner V, Babor M et al (2019) Synthesis of upper rim-double-bridged calix[4]arenes bearing seven membered rings and related compounds. RSC Adv 9:22017–22030. https://doi.org/10.1039/C9RA05075B
Slavík P, Krupička M, Eigner V et al (2019) Rearrangement of meta-bridged calix[4]arenes promoted by internal strain. J Organomet Chem 84:4229–4235. https://doi.org/10.1021/acs.joc.9b00107
Talotta C, Rosa MD, Soriente A et al (2019) Co-conformational mechanoisomerism in a calix[6]arene-based [2]rotaxane. Supramol Chem 31:62–68. https://doi.org/10.1080/10610278.2018.1529316
Hymel JH, Townsend J, Vogiatzis KD (2019) CO2 capture on functionalized calixarenes: a computational study. J Phys Chem A 123:10116–10122. https://doi.org/10.1021/acs.jpca.9b08670
Özkınalı S, Karayel A (2019) Synthesis, characterization, conformational equilibrium and intramolecular hydrogen bond analysis of novel azocalix[4]arenes including acryloyl moiety using DFT studies. J Mol Struct 1176:303–313. https://doi.org/10.1016/j.molstruc.2018.08.097
Echabaane M, Rouis A, Bonnamour I, Ben Ouada H (2013) Optical, electrical and sensing properties of β-ketoimine calix[4]arene thin films. Mater Chem Phys 141:781–789. https://doi.org/10.1016/j.matchemphys.2013.06.004
Dumazet-Bonnamour I, Halouani H, Oueslati F, Lamartine R (2005) Calixarenes for metal cations extraction. Comptes Rendus Chimie 8:881–891. https://doi.org/10.1016/j.crci.2005.02.004
Dennington RI, Keith T, Millam J GaussView Version 5.0.8. Semichem Inc, Shawnee
Shishkanova TV, Vatrsková L, Spálovská D et al (2020) Complexation of cathinones by 4-tert-butylcalix[4]arene tetra-acetate as a possible technique for forensic analysis. Forensic Toxicol 38:70–78. https://doi.org/10.1007/s11419-019-00489-8
Mammino L (2019) Five- and six-member bowl-shaped structures from acylphloroglucinols: an ab initio and DFT study. J Mol Model 26:13. https://doi.org/10.1007/s00894-019-4208-z
Shamova LI, Shamov GA, Antipin IS, Konovalov AI (2009) Modeling K+ and Ag+ complexation by thiacalix[4]arene amides using DFT: the role of intramolecular hydrogen bonding. J Phys Chem A 113:5691–5699. https://doi.org/10.1021/jp810947g
Mazzone G, Alberto ME, Ponte F et al (2018) Anion-π weak interactions in a heteroaromatic calixarene receptor. A theoretical investigation. Inorg Chim Acta 470:379–384. https://doi.org/10.1016/j.ica.2017.05.033
Gassoumi B, Ghalla H, R Ben C (2020) A theoretical study of the global and local electrophilicity, nucleophilicity, polarizability and QTAIM theory for calix[4]arene-gas interaction. Heliyon 6:e04554. https://doi.org/10.1016/j.heliyon.2020.e04554
Gassoumi B, Chaabene M, Ghalla H, Chaabane RB (2019) Role of hydrogen bonding interactions within of the conformational preferences of calix[n = 4,6,8]arene: DFT and QTAIM analysis. J Mol Model 26:12. https://doi.org/10.1007/s00894-019-4255-5
Mohan B, Modi K, Bhatia P et al (2018) An ionic receptor for Zn2+ metal ion using synthesised bis-formylpyrazole calix[4]arene and its computational study. Supramol Chem 30:589–599. https://doi.org/10.1080/10610278.2017.1415437
Bader RFW (1994) Atoms in molecules: a quantum theory; International Series of Monographs on Chemistry. Oxford University Press, Oxford
Biegler-König F, Schönbohm J (2002) Update of the AIM2000-program for atoms in molecules. J Comput Chem 23:1489–1494. https://doi.org/10.1002/jcc.10085
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Furer VL, Potapova LI, Chachkov DV et al (2020) Study of p-(3-carboxy-1-adamantyl)-calix[4]arene with hydrogen bonds along the upper and lower rim by IR spectroscopy and DFT. J Mol Model 26:179. https://doi.org/10.1007/s00894-020-04441-1
Furer VL, Vandyukov AE, Zaripov SR et al (2018) FT-IR and FT-Raman study of hydrogen bonding in p-alkylcalix[8]arenes. Vib Spectrosc 95:38–43. https://doi.org/10.1016/j.vibspec.2018.01.006
Acknowledgments
We thank to, Dr. Arzu Karayel and Dr. Sevil Özkınalı for the theoretical calculations of the calix[4]arene- N-β-ketoimine…Cu2+complexes. The numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, high performance, and Grid Computing Center (TRUBA resources).
Funding
The authors acknowledge financial support from the Tunisian’s Ministry of high education and scientific research.
Author information
Authors and Affiliations
Contributions
B. Gassoumi: wrote the paper, conceived and designed the analysis.
F.E. Ben Mohamed, N. Khedmi, M. Echabaane: performed a part of calculation and interpret some results.
A. Karayel, S. Özkınalı: analyzed and interpreted the data;
H. Ghalla: performed the AIM analysis.
R. Ben. Chaabane: supervisor of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Code availability
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1578 kb)
Rights and permissions
About this article
Cite this article
Gassoumi, B., Mohamed, F.E.B., Khedmi, N. et al. Theoretical assessment of calix[4]arene-N-β-ketoimine (n=1–4) derivatives: Conformational studies, optoelectronic, and sensing of Cu2+cation. J Mol Model 27, 16 (2021). https://doi.org/10.1007/s00894-020-04622-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04622-y