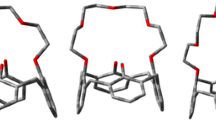
Abstract
Understanding the interactions of the cage molecules with a variety of invited molecules is getting very important. But, the hydrogen bonds can also play a crucial role in the interaction phenomenon. In this work, natural population analysis (NPA), chemical shifts, and atom in molecules (AIM) analysis have been used to identify the role of hydrogen bonds in the stability of CX[n] molecules. According to our calculation, the 13C NMR spectra are also sensitive to the nature of hydrogen bonds. We note that the DFT calculations have reproduced with a very good agreement, the experimentally observed chemical shifts of CX[4].




Similar content being viewed by others
References
Jacques V, Böhmer V (2012) Calixarenes: A versatile class of macrocyclic compounds. Springer Science a Business Media 3.
Haino T, Rudkevich DM, Shivanyuk A, Rissanen Jr K, Rebek Jr J (2000) Induced-fit molecular recognition with water-soluble cavitands. Chemistry – A European Journal 6:3797–3805. https://doi.org/10.1002/1521-3765(20001016)6:20<3797::AID-CHEM3797>3.0.CO;2-1
Ni X, Tomiyasu H, Shimizu T, Pérez-Casas C, Xi Z, Yamato T (2010) Synthesis and heteronuclear inclusion properties of a novel thiacalix[4]arene-based hard-soft receptor with 1,3-alternate conformation. J Incl Phenom Macrocycl Chem. 68:99–108. https://doi.org/10.1007/s10847-010-9740-5
Boulet B, Joubert L, Cote G, Bouvier-Capely C, Cossonnet C, Adamo C (2006) A combined experimental and theoretical study on the conformational behavior of a calix[6]arene. The Journal of Physical Chemistry. A. 110:5782–5791. https://doi.org/10.1021/jp0565305
Bourdelande JL, Font J, González-Moreno R, Nonell S (1998) Inclusion complex of calix[8] arene-C60: photophysical properties and its behaviour as singlet molecular oxygen sensitiser in the solid state. Journal of Photochemistry and Photobiology A: Chemistry. 115:69–71. https://doi.org/10.1016/S1010-6030(98)00245-7
Furer VL, Borisoglebskaya EI, Zverev VV, Kovalenko VI (2005) The hydrogen bonding and conformations of p-tert-butylcalix[4]arene as studied by IR spectroscopy and by DFT calculations. Spectrochim Acta A Mol Biomol Spectrosc. 62:483–493. https://doi.org/10.1016/j.saa.2005.02.001
Arena G, Contino A, Gulino FG, Magrí A, Sciotto D, Ungaro R (2000) Complexation of small neutral organic molecules by water soluble calix[4]arenes. Tetrahedron Letters 41:9327–9330. https://doi.org/10.1016/S0040-4039(00)01687-7
Nagarajan A, Ka J-W, Lee C-H (2001) Synthesis of expanded calix[n]pyrroles and their furan or thiophene analogues. Tetrahedron. 57:7323–7330. https://doi.org/10.1016/S0040-4020(01)00719-0
Kaneko S, Inokuchi Y, Ebata T, Aprà E, Xantheas SS (2011) Laser spectroscopic and theoretical studies of encapsulation complexes of calix[4]arene. J Phys Chem A. 115:10846–10853. https://doi.org/10.1021/jp204577j
Ebata T, Hontama N, Inokuchi Y, Haino T, Aprà E, Xantheas SS (2010) Encapsulation of Ar(n) complexes by calix[4]arene: endo- vs. exo-complexes. Phys Chem Chem Phys 12:4569–4579. https://doi.org/10.1039/b927441c
Lapenta R, Simone NAD, Buonerba A, Talotta C, Gaeta C, Neri P, Grassi A, Milione S (2018) Dinuclear zirconium complex bearing a 1,5-bridged-calix[8]arene ligand as an effective catalyst for the synthesis of macrolactones. Catal. Sci. Technol. 8:2716–2727. https://doi.org/10.1039/C7CY02537H
Brouwer, E.B., Enright, G.D., Ripmeester, J.A.: Solid-state NMR and diffraction studies ofp-tert-butylcalix[4]arene·nitrobenzene·xenon. Chem. Commun. 0, 939–940 (1997). doi:https://doi.org/10.1039/A607352B
Rudkevich DM (2007) Cover picture: progress in supramolecular chemistry of gases (Eur. J. Org. Chem. 20/2007). European Journal of Organic Chemistry 2007:3245–3245. https://doi.org/10.1002/ejoc.200790043
Gobre VV, Pinjari RV, Gejji SP (2010) Density functional investigations on the charge distribution, vibrational spectra, and NMR chemical shifts in cucurbit[n]uril (n = 5−12) Hosts. J. Phys. Chem. A. 114:4464–4470. https://doi.org/10.1021/jp100904c
Mohan N, Suresh CH (2014) Anion receptors based on highly fluorinated aromatic scaffolds. J Phys Chem A. 118:4315–4324. https://doi.org/10.1021/jp5019422
Mishra S, Suryaprakash N, Mishra SK, Suryaprakash N (2017) Intramolecular hydrogen bonding involving organic fluorine: NMR investigations corroborated by DFT-based theoretical calculations. Molecules. 22:423. https://doi.org/10.3390/molecules22030423
Pinjari RV, Khedkar JK, Gejji SP (2010) Cavity diameter and height of cyclodextrins and cucurbit[n]urils from the molecular electrostatic potential topography. J Incl Phenom Macrocycl Chem. 66:371–380. https://doi.org/10.1007/s10847-009-9657-z
Memon FN, Memon S, Memon S, Memon N (2011) Synthesis and application of a new calix[4]arene based impregnated resin for the removal of endosulfan from an aqueous environment. J. Chem. Eng. Data. 56:3336–3345. https://doi.org/10.1021/je2002732
Guan A-J, Zhang E-X, Xiang J-F, Li Q, Yang Q-F, Li L, Tang Y-L, Wang M-X (2011) Effects of loops and nucleotides in G-quadruplexes on their interaction with an azacalixarene, methylazacalix[6]pyridine. J. Phys. Chem. B. 115:12584–12590. https://doi.org/10.1021/jp204154m
Huggins DJ, Sherman W, Tidor B (2012) Rational approaches to improving selectivity in drug design. J. Med. Chem. 55:1424–1444. https://doi.org/10.1021/jm2010332
Muro S (2012) Challenges in design and characterization of ligand-targeted drug delivery systems. Journal of Controlled Release. 164:125–137. https://doi.org/10.1016/j.jconrel.2012.05.052
Sautrey G, Clarot I, Rogalska E, Regnouf-de-Vains J-B (2012) New potential prodrugs of aciclovir using calix[4]arene as a lipophilic carrier: synthesis and drug-release studies at the air–water interface. New J. Chem. 36:2060–2069. https://doi.org/10.1039/C2NJ40338B
Aksoy T, Erdemir S, Yildiz HB, Yilmaz M (2012) Novel water-soluble calix[4,6]arene appended magnetic nanoparticles for the removal of the carcinogenic aromatic amines. Water Air Soil Pollut. 223:4129–4139. https://doi.org/10.1007/s11270-012-1179-4
Wang L, Li L, Fan Y, Wang H (2013) Host–guest supramolecular nanosystems for cancer diagnostics and therapeutics. Advanced Materials. 25:3888–3898. https://doi.org/10.1002/adma.201301202
https://www.ccdc.cam.ac.uk/structures Cambridge Structural Database (CSD)
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al (2009) Gaussian, Inc, Wallingford CT
Dikmen G (2018) Experimental nuclear magnetic resonance spectral assignments, 1H/13C GIAO calculations, molecular structure and molecular resonance states of 4-Methyl-1H-Indazole-5-Boronic acid. Journal of Molecular Structure. 1156:136–145. https://doi.org/10.1016/j.molstruc.2017.11.097
Guzzo RN, Rezende MJC, Kartnaller V, de Carneiro JWM, Stoyanov SR, da Costa LM (2018) Experimental and DFT evaluation of the 1H and 13C NMR chemical shifts for calix[4]arenes. Journal of Molecular Structure 1157:97–105. https://doi.org/10.1016/j.molstruc.2017.12.038
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 112:8251–8260. https://doi.org/10.1021/ja00179a005
Pankratyev EY, Tulyabaev AR, Khalilov LM (2011) How reliable are GIAO calculations of 1H and 13C NMR chemical shifts? A statistical analysis and empirical corrections at DFT (PBE/3z) level. Journal of Computational Chemistry. 32:1993–1997. https://doi.org/10.1002/jcc.21786
Lee W, Balasubramanian P Advanced of NMR in biomedical field 322 (2016).
Dennington RI, Keith T, Millam J, GaussView Version 5.0.8. Semichem Inc.
Biegler-Konig F, Schonbohm J, Bayles DJ (2001). Comput. Chem 22
Shou W, Kang F, Huang S, Yan C, Zhou J, Wang Y (2019) Substituted aromatic-facilitated dissemination of mobile antibiotic resistance genes via an antihydrolysis mechanism across an extracellular polymeric substance permeable barrier. Environ. Sci. Technol. 53:604–613. https://doi.org/10.1021/acs.est.8b05750
Lu T, Chen F (2012) Multiwfn: A multifunctional wave function analyzer. Journal of Computational Chemistry. 33:580–592. https://doi.org/10.1002/jcc.22885
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters. 285:170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Acknowledgments
The authors acknowledge financial support from the Tunisian’s Ministry of Higher Education and Scientific Research. In this work, we were granted access to the HPC resources of the FLMSN, “Fédération Lyonnaise de Modélisation et Sciences Numériques,” partner of EQUIPEX EQUIP@MESO, and to the “Centre de calcul CC-IN2P3” at Villeurbanne, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict(s) of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1319 kb)
Rights and permissions
About this article
Cite this article
Gassoumi, B., Chaabene, M., Ghalla, H. et al. Role of hydrogen bonding interactions within of the conformational preferences of calix[n = 4,6,8]arene: DFT and QTAIM analysis. J Mol Model 26, 12 (2020). https://doi.org/10.1007/s00894-019-4255-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4255-5