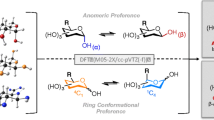
Abstract
The density functional theory method using the B3LYP/6-31G(d,p) level of theory was used to perform isoenergetic maps in order to determine the lower energy conformers of four disaccharides constituting alginic acids, which are based on β-D-mannuronic (M) and α-L-guluronic acid (G), called MM, GG, MG, and GM. The preferred structures are combined to monovalent (Li+, Na+, and K+) cations and further fully optimized, and an isoenergetic map corresponding to the complex (MG2−, 2Na+) was performed. Then, the reactivity of MG complexes with mono- and bivalent cations was studied using the global nucleophilic index. The position selectivity was also predicted using the local nucleophilic indices. It was demonstrated that experimental trends of relative reactivity and regioselectivity of the complexes are correctly predicted using these empirical indices of reactivity.

MM, GG, MG, and GM alginic acid disaccharides and reactivity of the MG metallic complexes







Similar content being viewed by others
References
Ribeiro ACF, Fabela I, Sobral AJFN, Verissimo LMP, Barros MCF, Rodrigo MM, Esteso MAE (2014) Diffusion of sodium alginate in aqueous solutions at T = 298.15 K. J Chem Thermodynam 74:263–268
Stewart MB, Gray SR, Vasiljevic T, Orbell JD (2014) Exploring the molecular basis for the metal-mediated assembly of alginate gels. Carbohydr Polym 102:246–253
Agulhon P, Robitzer M, Habas JJ, Quignard F (2014) Influence of both cation and alginate nature on the Rheological behavior of transition metal alginate gels. Carbohydr Polym 112:525–531
Deze EG, Papageorgiou SK, Favvas EP, Katsaros FK (2012) Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: effect of porosity in Cu2+ and Cd2+ ion sorption. Chem Eng J 209:537–546
Wang L-F, Shankar S, Rhim J-W (2017) Properties of alginate-based films reinforced with cellulose fibers and cellulose Nanowhiskers isolated from mulberry pulp. Food Hydrocoll 63:201–208
Stewart MB, Gray RS, Vasiljevic T, Orbell JD (2014) The role of poly-M and poly-GM sequences in the metal-mediated assembly of alginate gels. Carbohydr Polym 112:486–493
Atkins EDT, Isaac DH, Nieduszynski IA, Phelpst CF, Sheehan DK (1974) The polyuronides: their molecular architecture. Polymer 15:263–271
Crudales H, Larsen B, Smidsrqd O (1951) 13C-NMR studies of monomeric composition and sequence in alginate. Carbohydr Res 89:179–191
Davarci F, Turan D, Ozcelik B, Poncelet D (2017) The influence of solution viscosities and surface tension on calcium alginate microbead formation using dripping technique. Food Hydrocoll 62:119–127
Angelesc DG, Anastasescu M, Anghel DF (2014) Synthesis and modeling of calcium alginate nanoparticles inquaternary water-in-oil microemulsions. Colloids Surf A: Physicochem Eng Aspects 460:95–103
Menakbi C, Quignard F, Mineva T (2016) Complexation of trivalent metal cations to Mannuronate type alginate models from a density functional study. J Phys Chem B 120:3615–3623
Agulhon P, Robitzer M, David L, Quignard F (2012) Structural regime identification in ionotropic alginate gels: influence of the cation nature and alginate structure. Biomacromolecules 13:215–220
Agulhon P, Markova V, Robitzer M, Quignard F, Mineva T (2012) Structure of alginate gels: interaction of Diuronate units with divalent cations from density functional calculations. Biomacromolecules 13:1899–1907
Plazinski W, Drach M (2015) Binding of bivalent metal cations by α-L-guluronate: insights from the DFT-MD simulations. New J Chem 39:3987–3994
Ko YG, Lee HJ, Chun YJ, Choi US, Yoo KP (2013) Positive and negative electrorheological response of alginate salts dispersed suspensions under electric field. ACS Appl Mater Interfaces 5:1122–1130
Giammanco GE, Sosnofsky CT, Ostrowski AD (2015) Light-responsive iron(III)−polysaccharide coordination hydrogels for controlled delivery. ACS Appl Mater Interfaces 7:3068–3076
Taleb-Mokhtari IN, Rahal-Sekkal M, Vergoten G (2003) Modified UBFF calculations of the α-L-fucopyranose molecule in the crystalline state. Spectrochim Acta A 59:607–616
Sekkal N, Taleb-Mokhtari IN, Sekkal-Rahal M, Bleckmann P, Vergoten G (2003) Harmonic dynamics of α- and β-methyl-D-galactopyranoside in the crystalline state. Spectrochim Acta A 59:2883–2896
Fodil R, Sekkal-Rahal M, Sayede A (2017) Testing the CP correction procedure with different DFT methods on H-bonding complexes of κ-carrabiose with water molecules. J Mol Model 23:31
Berrekhchi-Berrahma-Bestaoui N, Derreumaux P, Sekkal-Rahal M, Springborg M, Sayede A, Yousfi N, Kadoun A (2013) Density functional conformational study of 2-O-sulphated 3,6 anhydro-α-D-galactose and of neo- κ- and ι-carrabiose molecules. J Mol Model 19(2):893–904
Yousfi N, Sekkal-Rahal M, Sayede A, Springborg M (2010) Relaxed energetic maps of κ-carrabiose: a DFT study. J Comput Chem 31:1312–1320
Bestaoui-Berrekhchi-Berrahma N, Sekkal-Rahal M, Derreumaux P, Yousfi N (2016) MP2 and DFT studies of β-D-neocarrabiose and β-D-neocarrabiose monohydrate. Comput Theo Chem 1091:24–30
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J Org Chem 73:4615–4624
Pérez P, Domingo LR, Aurell MJ, Contreras R (2003) Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 59:3117–3125
Quignard F, Valentinw R, Renzo DF (2008) Aerogel materials from marine polysaccharides. New J Chem 32:1300–1310
Wang N, Liu Q, Kang D, Gu J, Zhang W, Zhang D (2016) Facile self-crosslinking synthesis of 3D nanoporous Co3O4/carbon hybrid electrode materials for supercapacitors. ACS Appl Mater Interfaces 8(25):16035–16044
Kang D, Liu Q, Chen M, Gu J, Zhang D (2016) Spontaneous cross-linking for fabrication of nanohybrids embedded with size-controllable particles. ACS Nano 10:889–898
Zhang B, Yang H, Tang H, Hao G, Zhang Y, Deng S (2017) Insights into cryoprotective roles of carrageenan oligosaccharides in peeled whiteleg shrimp (Litopenaeus vannamei) during frozen storage. J Agric Food Chem 65(8):1792–1801
Brus J, Urbanova M, Czernek J, Pavelkova M, Kubova K, Vyslouzil J, Abbrent S, Konefal R, Horsky J, Vetchy D, Vyslouzil J, Kulich P (2017) Structure and dynamics of alginate gels cross-linked by polyvalent ions probed via solid state NMR spectroscopy. Biomacromolecules 18(8):2478–2488
Hossain KS, Miyanaga K, Maeda H, Nemoto N (2001) Sol-gel transition behavior of pure i-carrageenan in both salt-free and added salt states. Biomacromolecules 2:442–449
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci BG, Petersson A et al (2009) Gaussian09. Gaussian Inc, Wallingford
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:864–871
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:1133–1138
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377
Seal P, Jha PC, Chakrabarti S (2008) Static first order Hyperpolarizabilities of DNA base pairs: a configuration interaction study. J Mol Struc Theochem 855:64–68
Franzen PL, Zilio SC, Machado AEH, Madurro JM, Brito-Madurro AG, Ueno LT, Sampaio RN, Barbosa Neto NM (2008) Experimental and theoretical investigation of first Hyperpolarizability in Aminophenols. J Mol Struc 892:254–260
Mc Goverin CM, Walsh TJ, Gordon KC, Kay AJ, Woolhouse AD (2007) Predicting nonlinear optical properties in push–pull molecules based on methyl pyridinium donor and 3-cyano-5,5-dimethyl-2(5H)-furanylidene-propanedinitrile acceptor units using vibrational spectroscopy and density functional theory. Chem Phys Lett 443:298–303
Parr RG, Donnelly RA, Levy M, Palke W (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford
Koopmans TA (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a Nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct THEOCHEM 865:68–72
Parr RG, Yang W (1984) Density functional approach to the frontier-Electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050
Yang W, Mortier W (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711
Pérez P, Domingo LR, Duque-Noreña M, Chamorro E (2009) A condensed-to-atom nucleophilicity index. an application to the director effects on the electrophilic aromatic substitutions. J Mol Struct Theochem 895:86–91
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. J Chem Phys 23:1833–1840
Cossi M, Scalmani G, Rega N, Barone V (2002) New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J Chem Phys 117:43–54
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Braccini I, Peérez S (2001) Molecular basis of Ca2+-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules 2:1089–1096
Braccini I, Grasso RP, Pérez S (1999) Conformational and configurational features of acidic polysaccharides and their interactions with calcium ions: a molecular modeling investigation. Carbohydr Res 317:119–130
Dalheim MØ, Vanacker J, Najmi MA, Aachmann FL, Strand BL, Christensen BE (2016) Efficient functionalization of alginate biomaterials. Biomaterials 80:146–156
Yang J-S, Xie Y-J, He W (2011) Research progress on chemical modification of alginates: a review. Carbohydr Polym 84:33–39
Chattaraj PK, Sengupta S (1999) Chemical hardness as a possible diagnostic of the chaotic dynamics of Rydberg atoms in an external field. J Phys Chem A 103:6122–6126
Prabavathi N, Nilufer A, Krishnakumar V (2013) Spectroscopic (FT-IR, FT-Raman, UV and NMR) investigation, conformational stability, NLO properties, HOMO–LUMO and NBO analysis of hydroxyquinoline derivatives by density functional theory calculations. Spectrochim Acta Part A 114:449–474
Karnan M, Balachandran V, Murugan M, Murali MK, Nataraj A (2013) Vibrational (FT-IR and FT-Raman) spectra, NBO, HOMO–LUMO, molecular electrostatic potential surface and computational analysis of 4-(trifluoromethyl)benzylbromide. Spectrochim Acta Part A 116:84–95
Prabavathi N, Nilufer A, Krishnakumar V (2013) Vibrational spectroscopic (FT-IR and FT-Raman) studies, natural bond orbital analysis and molecular electrostatic potential surface of isoxanthopterin. Spectrochim Acta Part A 114:101–113
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bekri, L., Zouaoui-Rabah, M., Springborg, M. et al. A structural DFT study of MM, GG, MG, and GM alginic acid disaccharides and reactivity of the MG metallic complexes. J Mol Model 24, 312 (2018). https://doi.org/10.1007/s00894-018-3845-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3845-y