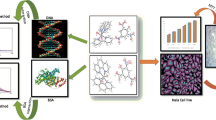
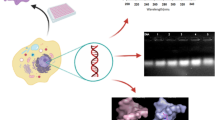
Abstract
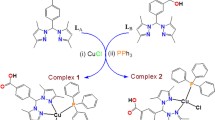
One iron(III) and two manganese(III) complexes based on thiosemicarbazone were synthesized and characterized using analytical and spectroscopic data. The crystallographic analysis showed the square pyramid structures of the complexes. Electronic spectra analysis was performed to determine the nature of the interaction between the complexes and calf thymus DNA (CT-DNA). DNA cleavage activities of the complexes were examined by gel electrophoresis (pBR322 DNA). The cytotoxicity of the complexes was determined against human cervical carcinoma (HeLa) and human colorectal adenocarcinoma (HT-29) cell lines by MTT assay. The results indicated that complex Fe1 is bound to CT-DNA via the intercalation mode, while complexes Mn1 and Mn2 are bound to CT-DNA via groove binding and/or electrostatic interactions rather than the intercalation mode. In addition, they showed good binding activity, which followed the order of Fe1 > Mn2 > Mn1. Complexes were found to promote the cleavage of DNA from supercoiled form (SC, Form I) to nicked circular form (NC, Form II) without concurrent formation of Form III, revealing the single-strand DNA cleavage. No significant cleavage was found in the presence of Mn1 and Mn2; however, it was observed at 2000 and 3000 µM concentrations of Fe1. The ability of Fe1 to cleave DNA was greater than that of other complexes and these results are in conformity with their DNA-binding affinities. Cytotoxicity determination tests revealed that the complex Fe1 on HeLa and HT-29 cells exhibited a higher anti-proliferative effect than Mn1 and Mn2 (Fe1 > Mn2 > Mn1). These studies suggested that the complex Fe1 could be a good candidate as a chemotherapeutic drug targeting DNA.
Graphical abstract






Similar content being viewed by others
References
Pahontu E, Julea F, Rosu T, Purcarea V, Chumakov Y, Petrenco P, Gulea A (2015) Antibacterial, antifungal and in vitro antileukaemia activity of metal complexes with thiosemicarbazones. J Cell Mol Med 19:865–878
Pelosi G, Bisceglie F, Bignami F, Ronzi P, Schiavone P, Re MC, Casoli C, Pilotti E (2010) Antiretroviral activity of thiosemicarbazone metal complexes. J Med Chem 53:8765–8769
da Silva JBP, do AF Navarro DM, da Silva AG, Santos GK, Dutra KA, Moreira DR, Ramos MN, Espíndola JWP, de Oliveira ADT, Brondani DJ (2015) Thiosemicarbazones as Aedes aegypti larvicidal. Eur J Med Chem 100:162–175
Dilworth JR, Hueting R (2012) Metal complexes of thiosemicarbazones for imaging and therapy. Inorg Chim Acta 389:3–15
Kunos CA, Chu E, Beumer JH, Sznol M, Ivy SP (2017) Phase I trial of daily triapine in combination with cisplatin chemotherapy for advanced-stage malignancies. Cancer Chemother Pharmacol 79:201–207
Mortazavi A, Ling Y, Martin LK, Wei L, Phelps MA, Liu Z, Harper EJ, Ivy SP, Wu X, Zhou B-S (2013) A phase I study of prolonged infusion of triapine in combination with fixed dose rate gemcitabine in patients with advanced solid tumors. Invest New Drugs 31:685–695
Ott I, Gust R (2007) Non platinum metal complexes as anti-cancer drugs. Arch Pharm 340:117–126
Patil SA, Patil SA, Patil R, Keri RS, Budagumpi S, Balakrishna GR, Tacke M (2015) N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs. Future Med Chem 7:1305–1333
Sangeetha S, Murali M (2018) Non-covalent DNA binding, protein interaction, DNA cleavage and cytotoxicity of [Cu(quamol)Cl].H2O. Int J Biol Macromol 107:2501–2511
Khan T, Dixit S, Ahmad R, Raza S, Azad I, Joshi S, Khan AR (2017) Molecular docking, PASS analysis, bioactivity score prediction, synthesis, characterization and biological activity evaluation of a functionalized 2-butanone thiosemicarbazone ligand and its complexes. J Chem Biol 10:91–104
Balachandran C, Haribabu J, Jeyalakshmi K, Bhuvanesh NS, Karvembu R, Emi N, Awale S (2018) Nickel (II) bis (isatin thiosemicarbazone) complexes induced apoptosis through mitochondrial signaling pathway and G0/G1 cell cycle arrest in IM-9 cells. J Inorg Biochem 182:208–221
Zhang H, Thomas R, Oupicky D, Peng F (2008) Synthesis and characterization of new copper thiosemicarbazone complexes with an ONNS quadridentate system: cell growth inhibition, S-phase cell cycle arrest and proapoptotic activities on cisplatin-resistant neuroblastoma cells. J Biol Inorg Chem 13:47–55
Rettondin AR, Carneiro ZA, Gonçalves AC, Ferreira VF, Oliveira CG, Lima AN, Oliveira RJ, de Albuquerque S, Deflon VM, Maia PI (2016) Gold (III) complexes with ONS-Tridentate thiosemicarbazones: toward selective trypanocidal drugs. Eur J Med Chem 120:217–226
Matesanz AI, Jimenez-Faraco E, Ruiz MC, Balsa LM, Navarro-Ranninger C, León IE, Quiroga AG (2018) Mononuclear Pd (ii) and Pt (ii) complexes with an α-N-heterocyclic thiosemicarbazone: cytotoxicity, solution behaviour and interaction versus proven models from biological media. Inorganic Chem Front 5:73–83
Park KC, Fouani L, Jansson PJ, Wooi D, Sahni S, Lane DJ, Palanimuthu D, Lok HC, Kovačević Z, Huang ML (2016) Copper and conquer: copper complexes of di-2-pyridylketone thiosemicarbazones as novel anti-cancer therapeutics. Metallomics 8:874–886
Bal T, Atasever B, Solakoğlu Z, Erdem-Kuruca S, Ülküseven B (2007) Synthesis, characterisation and cytotoxic properties of the N1, N4-diarylidene-S-methyl-thiosemicarbazone chelates with Fe(III) and Ni (II). Eur J Med Chem 42:161–167
Atasever B, Ülküseven B, Bal-Demirci T, Erdem-Kuruca S, Solakoğlu Z (2010) Cytotoxic activities of new iron (III) and nickel (II) chelates of some S-methyl-thiosemicarbazones on K562 and ECV304 cells. Invest New Drugs 28:421–432
Kaya B, Atasever-Arslan B, Kalkan Z, Gur H, Ulkuseven B (2016) Apoptotic mechanisms of nickel (II) complex with N1-acetylacetone-N4-4-methoxy-salicylidene-S-allyl-thiosemicarbazone on HL60 leukemia cells. Gen Physiol Biophys 35:451–458
Bal-Demirci T, Şahin M, Özyürek M, Kondakçı E, Ülküseven B (2014) Synthesis, antioxidant activities of the nickel (II), iron (III) and oxovanadium (IV) complexes with N2O2 chelating thiosemicarbazones. Spectrochim Acta Part A Mol Biomol Spectrosc 126:317–323
Kaya B, Şahin O, Bener M, Ülküseven B (2018) Iron (III) and nickel (II) complexes with S-alkyl (n-C1-6)-thiosemicarbazidato ligands: synthesis, structural characterization, and antioxidant features. J Mol Struct 1167:16–22
Yamazaki C (1975) The structure of isothiosemicarbazones. Can J Chem 53:610–615
Gradinaru J, Forni A, Simonov Y, Popovici M, Zecchin S, Gdaniec M, Fenton DE (2004) Mononuclear nickel (II) and copper (II) complexes with Schiff base ligands derived from 2, 6-diformyl-4-methylphenol and S-methylisothiosemicarbazones. Inorg Chim Acta 357:2728–2736
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Sheldrick GM (2015) SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Adv 71:3–8
Bruker S-N (2013) Data reduction software. Bruker AXS Inc, Madison, Wisconsin, USA
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Jvd Streek, Wood PA (2008) Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J Appl Crystallogr 41:466–470
Farrugia LJ (2012) WinGX and ORTEP for windows: an update. J Appl Crystallogr 45:849–854
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208-IN201
Gultneh Y, Khan AR, Blaise D, Chaudhry S, Ahvazi B, Marvey BB, Butcher RJ (1999) Syntheses and structures of and catalysis of hydrolysis by Zn(II) complexes of chelating pyridyl donor ligands. J Inorg Biochem 75:7–18
Li DD, Tian JL, Gu W, Liu X, Zeng HH, Yan SP (2011) DNA binding, oxidative DNA cleavage, cytotoxicity, and apoptosis-inducing activity of copper(II) complexes with 1,4-tpbd (N, N, N’, N’-tetrakis(2-yridylmethyl)benzene-1,4-diamine) ligand. J Inorg Biochem 105:894–901
Wolfe A, Shimer GH Jr, Meehan T (1987) Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 26:6392–6396
Thompson KH, Orvig C (2006) Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans 761–764
Pyle AM, Rehmann JP, Meshoyrer R, Kumar CV, Turro NJ, Barton JK (1989) Mixed-ligand complexes of ruthenium(II): factors governing binding to DNA. J Am Chem Soc 111:3051–3058
Jeyalakshmi K, Selvakumaran N, Bhuvanesh NSP, Sreekanth A, Karvembu R (2014) DNA/protein binding and cytotoxicity studies of copper(ii) complexes containing N, N′, N′′-trisubstituted guanidine ligands. RSC Adv 4:17179–17195
Borowska J, Sierant M, Sochacka E, Sanna D, Lodyga-Chruscinska E (2015) DNA binding and cleavage studies of copper(II) complexes with 2′-deoxyadenosine modified histidine moiety. JBIC 20:989–1004
Shahabadi N, Kashanian S, Darabi F (2009) In vitro study of DNA interaction with a water-soluble dinitrogen schiff base. DNA Cell Biol 28:589–596
Pravin N, Utthra PP, Kumaravel G, Raman N (2016) Effective DNA binding and cleaving tendencies of malonic acid coupled transition metal complexes. J Mol Struct 1123:162–170
Kulaksizoğlu S, Gökçe C, Güp R (2012) Synthesis and characterization of bis (azine) ligands and metal complexes: DNA-interaction and extraction properties for metals and dichromate anions. Turk J Chem 36:717–733
Netalkar PP, Netalkar SP, Budagumpi S, Revankar VK (2014) Synthesis, crystal structures and characterization of late first row transition metal complexes derived from benzothiazole core: anti-tuberculosis activity and special emphasis on DNA binding and cleavage property. Eur J Med Chem 79:47–56
Bernadou J, Pratviel G, Bennis F, Girardet M, Meunier B (1989) Potassium monopersulfate and a water-soluble manganese porphyrin complex,[Mn (TMPyP)](OAc) 5, as an efficient reagent for the oxidative cleavage of DNA. Biochemistry 28:7268–7275
Babu MS, Reddy KH, Krishna PG (2007) Synthesis, characterization, DNA interaction and cleavage activity of new mixed ligand copper (II) complexes with heterocyclic bases. Polyhedron 26:572–580
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Scheffler H, You Y, Ott I (2010) Comparative studies on the cytotoxicity, cellular and nuclear uptake of a series of chloro gold(I) phosphine complexes. Polyhedron 29:66–69
Rubner G, Bensdorf K, Wellner A, Bergemann S, Ott I, Gust R (2010) [Cyclopentadienyl]metal carbonyl complexes of acetylsalicylic acid as neo-anticancer agents. Eur J Med Chem 45:5157–5163
Ahmadi M, Mague JT, Akbari A, Takjoo R (2012) Dianion N1, N4-bis (salicylidene)-S-allyl-thiosemicarbazide complexes: synthesis, structure, spectroscopy and thermal behavior. Polyhedron 42:128–134
Kobayashi H, Yanagawa Y, Osada H, Minami S, Shimizu M (1973) Electronic spectra of high-spin iron (III) tetraphenylporphins. Bull Chem Soc Jpn 46:1471–1479
Habibi MH, Askari E (2013) Synthesis, structural characterization, thermal, and electrochemical investigations of a square pyramid manganese(III) complex with a Schiff base ligand acting as N2O2 tetradentate in equatorial and as O monodendate in axial positions: application as a precursor for preparation of Mn-doped ZnO nanoparticle. Synth React Inorg, Met-Org, Nano-Met Chem 43:406–411
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC (1984) Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J Chem Soc Dalton Trans 1349–1356
Tobón-Trujillo LM, Villanueva-Sánchez LF, Martínez-Otero D, Dorazco-González A (2015) Crystal structure of bis (μ2-tetrabromophthalato-κ2O1: O2) bis [aqua (N, N, N′, N′-tetramethylethane-1, 2-diamine-κ2 N, N′) copper (II)]. Acta Crystallogr Sect E Crystallogr Commun 71:m171–m172
Arjmand F, Mohani B, Ahmad S (2005) Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu (II) complex. Eur J Med Chem 40:1103–1110
Szczepanik W, Ciesiołka J, Wrzesiński J, Skała J, Jeżowska-Bojczuk M (2003) Interaction of aminoglycosides and their copper(ii) complexes with nucleic acids: implication to the toxicity of these drugs. Dalton Trans 1488–1494
Arjmand F, Sayeed F, Parveen S, Tabassum S, Juvekar AS, Zingde SM (2013) Design and synthesis of (S)- and (R)-enantiomers of [4-(2-hydroxy-1-phenylethylimino) pent-2-ol] dimethyltin (IV) and 2, 2-dimethyl-4-phenyl-1, 3, 2-oxazastannolidine: in vitro antitumor activity against human tumor cell lines and in vivo assay of (S)-enantiomers. Dalton Trans 42:3390–3401
Alizadeh R, Afzal M, Arjmand F (2014) In vitro DNA binding, pBR322 plasmid cleavage and molecular modeling study of chiral benzothiazole Schiff-base-valine Cu (II) and Zn (II) complexes to evaluate their enantiomeric biological disposition for molecular target DNA. Spectrochim Acta Part A Mol Biomol Spectrosc 131:625–635
Sudhamani CN, Naik HSB, Naik TRR, Prabhakara MC (2009) Synthesis, DNA binding and cleavage studies of Ni(II) complexes with fused aromatic N-containing ligands. Spectrochim Acta Part A Mol Biomol Spectrosc 72:643–647
Zhang G, Fu P, Wang L, Hu M (2011) Molecular spectroscopic studies of farrerol interaction with Calf Thymus DNA. J Agric Food Chem 59:8944–8952
Akdi K, Vilaplana RA, Kamah S, González-Vílchez F (2005) Effects of Tris and Hepes buffers on the interaction of palladium–diaminopropane complexes with DNA. J Inorg Biochem 99:1360–1368
Shahabadi N, Kashanian S, Fatahi A (2011) Identification of binding mode of a platinum (II) complex, PtCl(2)(DIP), and Calf Thymus DNA. Bioinorg Chem Appl 2011:687571
Li Q, Yang P, Wang H, Guo M (1996) Diorganotin(IV) antitumor agent. (C2H5)2SnCl2 (phen)/nucleotides aqueous and solid-state coordination chemistry and its DNA binding studies. J Inorg Biochem 64:181–195
Shi S, Liu J, Li J, Zheng KC, Huang XM, Tan CP, Chen LM, Ji LN (2006) Synthesis, characterization and DNA-binding of novel chiral complexes delta- and lambda-[Ru(bpy)2L]2 + (L = o-mopip and p-mopip). J Inorg Biochem 100:385–395
Jaumot J, Gargallo R (2012) Experimental methods for studying the interactions between G-quadruplex structures and ligands. Curr Pharm Des 18:1900–1916
Sun H, Xiang J, Liu Y, Li L, Li Q, Xu G, Tang Y (2011) A stabilizing and denaturing dual-effect for natural polyamines interacting with G-quadruplexes depending on concentration. Biochimie 93:1351–1356
Arjmand F, Parveen S, Afzal M, Toupet L, Hadda TB (2012) Molecular drug design, synthesis and crystal structure determination of Cu II–Sn IV heterobimetallic core: DNA binding and cleavage studies. Eur J Med Chem 49:141–150
Lazic D, Arsenijevic A, Puchta R, Bugarcic ZD, Rilak A (2016) DNA binding properties, histidine interaction and cytotoxicity studies of water soluble ruthenium(ii) terpyridine complexes. Dalton Trans (Cambridge, England: 2003) 45:4633–4646
Milutinović MM, Bugarčić ŽD, Wilhelm R (2018) A camphor based 1,3-diamine Ru(ii) terpyridine complex: synthesis, characterization, kinetic investigation and DNA binding. New J Chem 42:7607–7611
Tabassum S, Zaki M, Afzal M, Arjmand F (2014) Synthesis and characterization of Cu(II)-based anticancer chemotherapeutic agent targeting topoisomerase Iα: in vitro DNA binding, pBR322 cleavage, molecular docking studies and cytotoxicity against human cancer cell lines. Eur J Med Chem 74:509–523
Liang F, Wang P, Zhou X, Li T, Li Z, Lin H, Gao D, Zheng C, Wu C (2004) Nickel(II) and cobalt(II) complexes of hydroxyl-substituted triazamacrocyclic ligand as potential antitumor agents. Bioorg Med Chem Lett 14:1901–1904
Melvin MS, Tomlinson JT, Saluta GR, Kucera GL, Lindquist N, Manderville RA (2000) Double-Strand DNA Cleavage by Copper Prodigiosin. J Am Chem Soc 122:6333–6334
Arjmand F, Sharma GC, Muddassir M, Tabassum S (2011) Synthesis and enantiopreferential DNA-binding profile of late 3d transition metal R-and S-enantiomeric complexes derived from N, N-bis-(1-benzyl-2-ethoxyethane): validation of R-enantiomer of copper (II) complex as a human topoisomerase II inhibitor. Chirality 23:557–567
Tabassum S, Ahmad M, Afzal M, Zaki M, Bharadwaj PK (2014) Synthesis and structure elucidation of a copper(II) Schiff-base complex: in vitro DNA binding, pBR322 plasmid cleavage and HSA binding studies. J Photochem Photobiol B 140:321–331
Deng J, Su G, Chen P, Du Y, Gou Y, Liu Y (2018) Evaluation of DNA binding and DNA cleavage of nickel(II) complexes with tridentate α-N-heterocyclic thiosemicarbazones ligands. Inorg Chim Acta 471:194–202
Fu XB, Liu DD, Lin Y, Hu W, Mao ZW, Le XY (2014) Water-soluble DNA minor groove binders as potential chemotherapeutic agents: synthesis, characterization, DNA binding and cleavage, antioxidation, cytotoxicity and HSA interactions. Dalton Trans (Cambridge, England: 2003) 43:8721–8737
Alizadeh R, Yousuf I, Afzal M, Srivastav S, Srikrishna S, Arjmand F (2015) Enantiomeric fluoro-substituted benzothiazole Schiff base-valine Cu (II)/Zn (II) complexes as chemotherapeutic agents: DNA binding profile, cleavage activity, MTT assay and cell imaging studies. J Photochem Photobiol B 143:61–73
Kumari R, Nath M (2017) Tri- and diorganotin(IV) derivatives of non-steroidal anti-inflammatory drug sulindac: characterization, electronic structures (DFT), DNA binding and plasmid cleavage studies. Appl Organomet Chem 31:e3661
Annaraj B, Balakrishnan C, Neelakantan MA (2016) Synthesis, structure information, DNA/BSA binding affinity and in vitro cytotoxic studies of mixed ligand copper(II) complexes containing a phenylalanine derivative and diimine co-ligands. J Photochem Photobiol, B 160:278–291
Manan MAFA, Tahir MIM, Crouse KA, Rosli R, How FN-F, Watkin DJ (2011) The crystal structure and cytotoxicity of centrosymmetric copper(II) complex derived from S-methyldithiocarbazate with Isatin. J Chem Crystallogr 41:1866–1871
Sriwiriyajan S, Ninpesh T, Sukpondma Y, Nasomyon T, Graidist P (2014) Cytotoxicity screening of plants of genus piper in breast cancer cell lines. Trop J Pharmaceut Res 13:921–928
Gren R (1972) Protocol for screening chemical agents and natural product against animal tumors and other biological system. Cancer Chemother Rep 3:51–61
Acknowledgements
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University-Cerrahpasa. The authors acknowledge Scientific and Technological Research Application and Research Center, Sinop University, Turkey, for the use of the Bruker D8-QUEST diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2019_1653_MOESM1_ESM.pdf
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 1877123 for Fe1 and 1877124 for Mn1. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk) (PDF 180 kb)
Rights and permissions
About this article
Cite this article
Kaya, B., Yılmaz, Z.K., Şahin, O. et al. Structural analysis and biological functionalities of iron(III)– and manganese(III)–thiosemicarbazone complexes: in vitro anti-proliferative activity on human cancer cells, DNA binding and cleavage studies. J Biol Inorg Chem 24, 365–376 (2019). https://doi.org/10.1007/s00775-019-01653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01653-6