Abstract
Typical derivatization reagents for saccharides in high-performance thin-layer chromatography, like 2-naphthol sulfuric acid, aniline diphenylamine orthophosphoric acid, or p-aminobenzoic acid, generally detect both reducing and non-reducing saccharides. A new reagent was found with ethylamine, specifically reacting with reducing saccharides on normal-phase silica gel plates, resulting in strongly fluorescent zones after heating the plate at 150 °C for 15 min. In contrast, non-reducing saccharides generally did not reveal fluorescent signals tested with 26 different saccharides. Optimal chromatographic separation was achieved with a mixture of 2-propyl acetate, methanol, and water with 1 mg/mL natural product reagent A when the plate was twofold developed. The high sensitivity of the ethylamine derivatization was shown with mean limits of detection and quantification of 10 and 30 ng per zone, respectively, calculated by different methods for selected mono- and disaccharides. The developed method has exemplarily been used for the digestion control of starch by α-amylase, the determination of lactose in lactose-free milk, and for the quantitative and qualitative study of honey. The analysis of honey gave an excellent example of the advantageous consecutive derivatization with ethylamine and aniline diphenylamine orthophosphoric acid reagent as reagent sequence to detect the coelution of reducing and non-reducing saccharides.
Similar content being viewed by others
1 Introduction
Saccharides typically lack suitable chromophores or fluorophores, making selective and specific chromatographic analysis by high-performance liquid column chromatography (HPLC) impossible, except for electrochemical [1] or mass-selective [2] detection. In contrast, analysis by high-performance thin-layer chromatography (HPTLC) has the great advantage of highly selective derivatizations of saccharides, as has been thoroughly and very recently reviewed [3]. Therefore, only a few aspects should be mentioned. The aniline diphenylamine orthophosphoric acid reagent was widely used, providing different colors supporting the identification of saccharides in addition to their relative migration distances [4,5,6,7,8]. Additionally, aniline diphenylamine orthophosphoric acid reagent resulted in a sucralose-specific fluorescent signal, while other saccharides did not [5]. The colorful differentiation of saccharides is not observed with the 2-naphthol sulfuric acid reagent, generally resulting in brownish zones, but this reagent is well equivalent in terms of sensitivity [5, 9, 10]. p-Aminobenzoic acid, resulting in strongly fluorescent zones of saccharides, clearly outperforms the former reagents in sensitivity [11, 12]. Identical reagent-free fluorescent signals were obtained by heating the chromatogram developed on a silica gel amino plate [5, 13, 14].
All reagents used so far have in common that they detect both reducing and non-reducing saccharides equally. In 1904, Wöhlk reported a color reaction of lactose and maltose, which gave a specific deep red color when heated in the presence of aqueous ammonia, while other reducing saccharides gave only faint yellow solutions [15]. However, non-reducing saccharides like sucrose and raffinose did not result in any color. Forty years later, Fearon replaced ammonia with methylamine or ethylamine and observed comparable color reactions [16]. These old color reactions encouraged us to study the suitability of simple amines for the specific detection of saccharides on normal-phase silica gel plates.
2 Experimental
2.1 Chemicals and materials
l-Arabinose (Ara), d-cellobiose (Cel), l-fucose (Fuc), d-fructose (Fru), d-galactose (Gal), N-acetyl-d-galactosamine (GalNAc), d-glucose (Glc), N-acetyl-d-glucosamine (GlcNAc), d-mannose (Man), d-melibiose monohydrate (Mel), d-raffinose pentahydrate (Raf), l-rhamnose (Rha), d-ribose (Rib), l-sorbose (Sor), d-sucrose (Suc), and d-trehalose (Tre) (all saccharides ≥ 98%), acetonitrile (HPLC grade), 2-propyl acetate (> 99%), methanol (HPLC grade), natural product reagent A (≥ 98%), sodium dihydrogenphosphate monohydrate (> 98%), and sodium chloride (> 99.5%) were delivered by Carl Roth (Karlsruhe, Germany). d-galacturonic acid (GalU), d-glucosamine hydrochloride (GlcN), sodium d-glucose-6-phosphate (GlcP), d-lactose monohydrate (Lac), d-melezitose monohydrate (Mlz), d-sorbitol (Sol), stachyose monohydrate (Sta), sucralose (Sul) (all saccharides ≥ 98%), ethylamine (aqueous solution 70%), and α-amylase from human saliva (Type IX-A, lyophilized powder, 1,000–3,000 units/mg protein) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Purified water was prepared by a Destamat Bi 18E (Heraeus, Hanau, Germany). HPTLC plates silica gel 60 were from Merck (Darmstadt, Germany). Wheat starch, wheat flour, spring honey, summer honey, blossom honey, and the lactose-free milk samples were purchased from a local supermarket.
2.2 Standard solutions
Saccharide stock solutions were individually prepared in water (10 mg/mL, stored at 4 °C for maximal one week) and freshly diluted with water to 1 mg/mL to obtain individual working standard solutions. Standard mixes for quantifications (0.1 mg/mL) were prepared by pipetting 100 µL of the respective working standard solutions, filled up to 1000 µL with water.
2.3 Sample preparation
For the digestion of starches, 100 mg of soluble starch, wheat starch, and wheat flour were weighed into 25-mL falcon tubes and suspended in 10 mL of buffer (20 mM sodium dihydrogen phosphate with 6.7 mM sodium chloride, pH 6.7). The falcon tubes were placed in a boiling water bath for 15 min to inactivate enzymes. A total of 1 mL of each freshly vortexed suspension was pipetted into a 2-mL Eppendorf tube, followed by the addition of 1 U α-amylase; except for native samples (0 min). Incubation was carried out at ambient temperature for 1, 3, 5, 7, and 9 min using a Vortex Genie 2 with multi-tube holder (Scientific Industries, Bohemia, NY, USA) at speed 10. After incubation, α-amylase was deactivated in a boiling water bath for 15 min. The cooled samples were made up to a total volume of 2 mL with water, vortexed, and centrifuged at 17,000g (Heraeus Pico 17, Thermo Fisher Scientific, Schwerte, Germany).
Milk samples (100 µL) were pipetted into a 10-mL volumetric flask and filled up with methanol. After vortexing, the flask was allowed to stand for 10 min to settle down the proteins, whereafter the supernatant was filtrated through a 0.45-μm membrane filter (cellulose acetate) into an HPTLC vial.
Honey samples (1 g) were weighed into 25-mL falcon tubes, followed by the addition of 10 mL of water. After 15 min on a vortex with multi-tube holder at speed 10, the sample solutions were filtrated through a 0.45-μm membrane filter (cellulose acetate) into a 2-mL Eppendorf tube. For the qualitative and quantitative analyses, the honey solutions were diluted 1:25 and 1:250, respectively, with water in an HPTLC vial.
2.4 High-performance thin-layer chromatography
HPTLC instrumentation (CAMAG, Muttenz, Switzerland) consisted of Automatic TLC Sampler (ATS 4), Automatic development chamber (ADC 2), Derivatizer, TLC Visualizer 2, TLC Scanner 4, and Plate Heater III. The instruments were controlled by visionCATS software version 3.2 SP2. HPTLC silica gel 60 plates were prewashed with methanol and dried for 10 min in an oven at 110 °C. Samples and standard solutions were applied as 6‑mm bands with a track distance of 7 mm, distance from the lower edge 8 mm, and left edge minimal distance of 12 mm with the following settings: water as sample solvent type, standard (qualitative analyses) or quantification (quantitative analyses) as filling/rinsing quality, and 25 µL syringe volume. During the method development, standard solutions (1 mg/mL) were applied at 0.2 µL. For quantifications, standard solutions (0.1 mg/μL) were applied at 0.2, 0.5, 0.8, 1.4, and 2.0 μL. Starch samples were applied at 6 µL, milk samples at 1.0, 3.0, and 5.0 µL, and honey samples at 0.5 μL and 1.5 μL (1:25 and 1:250 dilutions).
After plate preconditioning for 20 min in a 20 cm × 10 cm twin-trough chamber (using filter paper and 10 mL mobile phase in the opposite trough), the plate was developed with 10 mL 2-propyl acetate–methanol–water (7:3.5:1.5, V/V) containing 1 mg/mL natural product reagent A up to 60 mm, taking 12 min, and thereafter dried inside a fume hood for 15 min. A second development was performed analogously. Alternatively, development was performed in the ADC 2 with chamber saturation (using filter paper) for 5 min and plate preconditioning for 15 min with 20 mL and 10 mL mobile phase, respectively, followed by plate drying for 10 min. Relative humidity of the surrounding air during experiments was < 50%.
Derivatization was carried out with 1 mL ethylamine solution (15% in water, Derivatizer, yellow nozzle, level 2), followed by plate heating at 150 °C for 15 min. Chromatograms were captured under 366 nm followed by fluorescence measurements (366 nm > 400 nm, measurement slit 5 mm × 0.2 mm). An optional second derivatization was performed with 2 mL aniline diphenylamine orthophosphoric acid reagent (yellow nozzle, level 5), followed by plate heating at 110 °C for 10 min. Chromatograms were documented at white light illumination (transmittance mode).
2.5 Validation
Limit of detection (LOD) and limit of quantification (LOQ) were determined for glucose-6-phosphate, maltose, lactose, fructose, and rhamnose. The standard solutions (0.1 mg/mL) were applied with 0.2, 0.5, 0.8, 1.4, and 2.0 µL (20–200 ng), followed by development, derivatization, and fluorescence measurement. The calculations followed the Deutsches Institut für Normung (DIN) method [17], the International Council for Harmonisation (ICH) guidelines [18], and the United States Pharmacopeia (USP) procedure [19] (available in visionCATS).
3 Results
3.1 Selectivity of ethylamine for reducing saccharides and reagent sequence
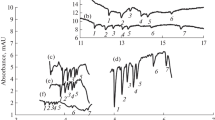
Both ethylamine and methylamine are available as aqueous solutions and can be used for derivatization by simple dilution. However, the vapor pressure of ethylamine (114 kPa at 20 °C) is three times lower than the vapor pressure of methylamine (300 kPa at 20 °C), which is why ethylamine was selected for the present study. The first experiments quickly showed that reducing mono- and disaccharides on the silica gel plate formed a dye, which was absent with the non-reducing sucrose (Fig. 1a). However, lactose and maltose did not give the described red color [16]. Instead, all reducing saccharides showed a non-specific ochre color, and this was only detectable when relatively high amounts (> 500 ng) were applied. This initially disappointing result turned positive when the plate was viewed under 366 nm illumination. Except for sucrose, the exemplarily applied saccharides revealed strongly fluorescent zones (Fig. 1b) comparable with the p-aminobenzoic acid reagent. The non-reducing sucrose could be detected after consecutive derivatization with the aniline diphenylamine orthophosphoric acid reagent reagent (Fig. 1c). For these promising saccharide-specific derivatizations, the optimal conditions for the detection and separation of saccharides were evaluated.
HPTLC–Vis/FLD chromatograms of glucose (Glc), fructose (Fru), lactose (Lac), sucrose (Suc), maltose (Mal), and a mixture of them (each 500 ng/band), on HPTLC silica gel 60 plates developed once with 2-propyl acetate–methanol–water (7:3.5:1.5, V/V) with 1 mg/mL natural product reagent A. Plate images were captured after derivatization with ethylamine under white light (a) and 366 nm illumination (b) as well as after consecutive derivatization with aniline diphenylamine orthophosphoric acid reagent under white light illumination (c)
3.2 Screening of 26 reducing and non-reducing saccharides
The optimal ethylamine concentration (15%), reagent volume (1 mL, applied via the Derivatizer), and heating temperature (150 °C) were determined (data not shown). Volumes > 1.5 mL of the aqueous reagent resulted in zone broadening due to a too-wet layer. The new derivatization reagent was validated with 26 reducing and non-reducing saccharides, including acids and amines. As a mobile phase, acetonitrile–water (4:1, V/V) [20] containing 1 mg/mL natural product reagent A [21,22,23], improving the separation, especially of fructose and glucose, was first tested. This planar hydrophilic interaction liquid chromatography has a short development time and results in the separation of the most saccharides tested (Fig. 2a) compared with the widely used mixtures of n-butanol–2-propanol–acetic acid. Again, all reducing saccharides showed a nice fluorescence, while the non-reducing saccharides, like stachyose, melezitose, trehalose, sucralose, and sucrose, did not. The exemplarily applied sugar alcohol sorbitol was also not detectable. But with increasing hRF, the zones became broader, and galacturonic acid migrated into the front with this solvent system. Therefore, mobile phase mixtures of 2-propyl acetate, methanol, and water [7] were studied in different ratios, also allowing rapid development. The optimum was found for a mixture of 7:3.5:1.5 (V/V) when the natural product reagent A was added at 1 mg/mL, and the plate was developed two-fold (Fig. 2b). By the second development with the same mobile phase, the zones became sharper, galacturonic acid now showed an hRF of 45, glucose and fructose were still separated, and N-acetylglucosamine and N-acetylgalactosamine more clearly provided double zones, most likely diastereomers.
HPTLC–FLD chromatograms of 26 saccharides (listed in section 2.1; 1 mg/mL, 0.2 μL applied) on HPTLC silica gel 60 plates developed with acetonitrile–water (4:1, V/V) containing 1 mg/mL natural product reagent A (a) and two-fold developed with 2-propyl acetate– methanol–water (7:3.5:1.5, V/V) with 1 mg/mL natural product reagent A (b) detected at 366 nm after derivatization with ethylamine
Generally, the separation of maltose and cellobiose could not be achieved, and the separation of both the pentoses and hexoses could be more satisfactory. But regarding the strong blue fluorescence, these monosaccharides seem overloaded at 200 ng per zone and lower amounts applied would provide better selectivity. However, separating such a number of saccharides is generally challenging. Appropriate adjustment of the mobile phase can be made for samples where specific separations are essential.
3.3 Limits of detection and quantification
The LOD and LOQ were exemplarily determined for glucose-6-phosphate, maltose, glucose, lactose, fructose, and rhamnose to cover the whole hRF range using derivatization via the ethylamine reagent. Depending on the calculation method, LODs ranged 6–10 ng per zone and LOQs ranged 14–30 ng per zone (Table 1), which is quite comparable with the literature [5, 11, 12].
3.4 Applications
As proof of principle, the presented method has exemplarily been used for the digestion control of starch by α-amylase, the detection of saccharides in lactose-free milk, and in the quantitative and qualitative analysis of honey.
3.4.1 Saccharide analysis of digested starch
The time course of enzymatic starch degradation was simply studied in vials with different starch sources, soluble starch, wheat starch, and wheat flour. Alternatively to in-vial degradation, an all-in-one nanoGIT system can be used, recently reported for the simulated static oral and intestinal on-surface amylolysis of flours and soluble starch [24]. To check for the presence of starch degradation products initially present in the samples, native sample extracts without adding α-amylase were also applied. The influence of enzymes in wheat flour was excluded due to the sample preparation (heating step). Starch degraded mainly into maltose (hRF 23) besides other oligosaccharides over time (Fig. 3). The most intense zones of maltose were found in the wheat flour, followed by the soluble starch and wheat starch. However, besides traces of glucose (hRF 35), maltose was also detected in the blank wheat flour extracts, naturally present in the flour.
HPTLC–FLD chromatograms of the saccharide analysis of digested starch samples, soluble starch (sS), wheat starch (WS), and wheat flour (WF) along with standard mixture (rhamnose, fructose, glucose, maltose, and glucose-6-phosphate with decreasing hRF, each 0.1 mg/mL, applied at 0.2, 0.5, 0.8, 1.4, and 2.0 μL as S1–S5) and blank (B, buffer used for digestion). The plate was two-fold developed with 2-propyl acetate–methanol–water (7:3.5:1.5, V/V) containing 1 mg/mL natural product reagent A, and detected at 366 nm after derivatization with ethylamine
3.4.2 Saccharide analysis of lactose-free milk
The HPTLC analysis of lactose-free milk was already reported with different reagents for derivatization on silica gel plates, whereby the p-aminobenzoic acid was found to perform best [11]. Three commercial milk samples labeled lactose-free (< 0.1%) were analyzed with the present method for their lactose content. In all samples, lactose (hRF 19) was visually almost slightly detectable at the highest application volume (5 µL), while galactose and glucose expectedly were strongly detected (Fig. 4). Thus, without densitometric measurement, the lactose concentration of the three milk samples was rated below the lowest lactose standard applied (20 ng per band), resulting in < 40 mg/100 mL lactose in milk, thus fulfilling the declarations.
HPTLC–FLD chromatograms of the saccharide analysis in lactose-free milk samples M1, M2, M3 (applied with 1, 3, and 5 μL) along with standard mixture (rhamnose, galactose, and lactose, decreasing hRF, each 0.1 mg/mL, applied at 0.2, 0.5, 0.8, 1.4, and 2.0 μL) and blank (B, methanol used for milk dilution). The plate was two-fold developed with 2-propyl acetate–methanol–water (7:3.5:1.5, V/V) containing 1 mg/mL natural product reagent A, and detected at 366 nm after derivatization with ethylamine
3.4.3 Saccharide analysis of honey
HPTLC methods for analyzing saccharides in honey or nectars were also already reported [4, 6]. With several different saccharides, honey is particularly well suited for demonstrating the selectivity of the ethylamine derivatization method. The main saccharides found in honey were glucose, fructose, melezitose, sucrose, and maltose [25]. Because only reducing saccharides can be detected with ethylamine, the two non-reducing saccharides (melezitose and sucrose) in the honey samples were detected first when the aniline diphenylamine orthophosphoric acid reagent was secondly applied as reagent sequence on the same plate.
Fructose (hRF 39) and glucose (hRF 35) were significantly present in all samples, and the tracks were overloaded with the aim also to record the saccharides that were only present in small amounts (Fig. 5a). Maltose (hRF 22) was only detectable in the spring and blossom honey samples but not in summer honey. Furthermore, another fluorescent zone was visible in all honey samples directly above maltose. After derivatization with aniline diphenylamine orthophosphoric acid reagent, a relatively weak zone was detected in the honey samples at the same hRF as sucrose in the standard mixture (Fig. 5b). However, the fluorescent zone in the honey samples (Fig. 5a, hRF 28) could not be assigned to sucrose because the sucrose standard is not expected to show fluorescence when derivatized with ethylamine.
Saccharide analysis of spring honey (SpH), summer honey (SuH), and blossom honey (BlH), diluted 1:250 and applied at 0.5 and 1.5 μL, along with standard mixture (fructose, glucose, sucrose, maltose, galactose, and melezitose, decreasing hRF, each 0.1 mg/mL, applied at 0.2, 0.5, 0.8, 1.4, and 2.0 μL, S1–S5) and blank (B, water used for sample dilution). The plate was two-fold developed with 2-propyl acetate–methanol–water (7:3.5:1.5, V/V) containing 1 mg/mL natural product reagent A, and detected at 366 nm after derivatization with ethylamine (a) and under white light illumination after consecutive derivatization with aniline diphenylamine orthophosphoric acid reagent (b)
On derivatization with ethylamine, below maltose, both the summer honey and the blossom honey slightly revealed another zone of a saccharide (hRF 18) not present in the standard mixture. At the same hRF, the plate gave a signal for melezitose after derivatization with aniline diphenylamine orthophosphoric acid reagent. Since melezitose is also a non-reducing saccharide, it cannot be this saccharide. In both cases, the reducing turanose, formed by hydrolysis of melezitose, could be a candidate to be proven with an appropriate standard. Still, it could not be excluded that sucrose and melezitose were additionally present in these samples. Nevertheless, this coelution would not have been detected with aniline diphenylamine orthophosphoric acid reagent alone as a reagent for derivatization, giving a nice example of the advantageous consecutive derivatization as reagent sequence as presented. In sample dilutions of 1:250, glucose and fructose could easily be quantified in the studied honey samples (Table 2).
4 Conclusion
Ethylamine was shown not to produce colorful zones of saccharides separated on silica gel plates, but ochre color and in particular brightly blue fluorescent zones specific for reducing saccharides, allowing highly sensitive detection at 366 nm. The preparation of the ethylamine reagent was simple and comparatively inexpensive and sustainable, since the concentrated aqueous solution only needed to be diluted with water. Exploiting a reagent sequence, non-reducing saccharides were additionally detectable after a consecutive derivatization with aniline diphenylamine orthophosphoric acid reagent on the same plate. The wide range of use of ethylamine for the specific detection of reducing saccharides was demonstrated via amylolysis of starches as well as analysis of lactose-free milk and honey. Advantageously, it also could be used to determine reducing saccharides without interference from a large excess of sucrose as a prominent saccharide in food.
References
Hanko VP, Rohrer JS (2000) Determination of carbohydrates, sugar alcohols, and glycols in cell cultures and fermentation broths using high-performance anion-exchange chromatography with pulsed amperometric detection. Anal Biochem 283:192–199. https://doi.org/10.1006/abio.2000.4653
Feil R, Lunn JE (2018) Quantification of soluble sugars and sugar alcohols by LC–MS/MS. In: António C (ed) Plant metabolomics: methods and protocols. Springer, New York, pp 87–100
Poole CF (2023) Chapter 15: Application of thin-layer chromatography to the analysis of saccharides. In: Poole CF (ed) Instrumental thin-layer chromatography. Handbooks in separation science, 2nd edn. Elsevier, Amsterdam, pp 413–435
Ruppel A, Morlock GE (2015) Content of carbohydrates in tropical rainforest nectars of marantaceae using high-performance thin-layer chromatography. J Planar Chromatogr 28:162–166. https://doi.org/10.1556/JPC.28.2015.2.13
Morlock G, Vega-Herrera M (2007) Two new derivatization reagents for planar chromatographic quantification of sucralose in dietetic products. J Planar Chromatogr 20:411–417. https://doi.org/10.1556/JPC.20.2007.6.4
Islam MK, Sostaric T, Lim LY, Hammer K, Locher C (2020) A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. J Planar Chromatogr 33:489–499. https://doi.org/10.1007/s00764-020-00054-9
Morlock GE, Gamlich F (2012) Analysis of biopolymers-the fingerprint of plants’ polysaccharides used as thickening agents. J Planar Chromatogr 25:244–250. https://doi.org/10.1556/JPC.25.2012.3.10
Aranda MB, Vega MH, Villegas RF (2005) Routine method for quantification of starch by planar chromatography (HPTLC). J Planar Chromatogr 18:285–289. https://doi.org/10.1556/JPC.18.2005.4.6
Cheong K-L, Li J, Zhao J, Li S-P (2014) A simple analysis of fructooligosaccharides in two medicinal plants by high-performance thin-layer chromatography. J Planar Chromatogr 27:245–250. https://doi.org/10.1556/JPC.27.2014.4.2
Popovic N, Fried B, Sherma J (2013) Effects of increased salinity on glucose and maltose composition of biomphalaria glabrata snails infected with schistosoma mansoni as determined by high-performance thin-layer chromatography-densitometry. J Planar Chromatogr 26:137–140. https://doi.org/10.1556/JPC.26.2013.2.6
Morlock GE, Morlock LP, Lemo C (2014) Streamlined analysis of lactose-free dairy products. J Chromatogr A 1324:215–223. https://doi.org/10.1016/j.chroma.2013.11.038
Morlock GE, Sabir G (2011) Comparison of two orthogonal liquid chromatographic methods for quantitation of sugars in food. J Liq Chromatogr Relat Technol 34:902–919. https://doi.org/10.1080/10826076.2011.571118
Klaus R, Fischer W, Hauck HE (1990) Application of a thermal in situ reaction for fluorimetric detection of carbohydrates on NH2-layers. Chromatographia 29:467–472. https://doi.org/10.1007/BF02261396
Morlock GE, Prabha S (2007) Analysis and stability of sucralose in a milk-based confection by a simple planar chromatographic method. J Agric Food Chem 55:7217–7223. https://doi.org/10.1021/jf071719u
Wöhlk A (1904) Über eine neue Reaktion auf Milchzucker (und Maltose). Anal Bioanal Chem 43:670–679. https://doi.org/10.1007/BF01520629
Fearon WR (1942) The detection of lactose and maltose by means of methylamine. Analyst 67:130. https://doi.org/10.1039/an9426700130
Deutsches Institut für Normung (2008) DIN 32645:2008–11. Chemical analysis: decision limit, detection limit and determination limit under repeatability conditions—terms, methods, evaluation. Beuth, Berlin
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2005) Validation of Analytical Procedures: Text and Methodology Q2(R1). Geneva. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
United States Pharmacopeia (2023) General Chapter 〈1210〉 Statistical Tools for Procedure Validation Validation, USP-NF. United States Pharmacopeial Convention, Rockville, MD
Morlock GE, Heil J (2020) HI-HPTLC-UV/Vis/FLD-HESI-HRMS and bioprofiling of steviol glycosides, steviol, and isosteviol in Stevia leaves and foods. Anal Bioanal Chem 412:6431–6448. https://doi.org/10.1007/s00216-020-02618-4
Kirchert S, Morlock GE (2018) Simultaneous determination of mono-, di-, oligo- and polysaccharides via planar chromatography in 4 different prebiotic foods and 60 naturally degraded inulin samples. J Chromatogr A 1569:212–221. https://doi.org/10.1016/j.chroma.2018.07.042
Pukl M, Prosek M (1990) Rapid quantitative TLC analysis of sugars using an improved commonly used solvent system. J Planar Chromatogr 1:173–176
Simonovska B (2000) Determination of inulin in foods. J AOAC Int 83:675–678. https://doi.org/10.1093/jaoac/83.3.675
Müller I, Morlock GE (2024) Quantitative saccharide release of hydrothermally treated flours by validated salivary/pancreatic on-surface amylolysis (nanoGIT) and high-performance thin-layer chromatography. Food Chem 432:137145. https://doi.org/10.1016/j.foodchem.2023.137145
Pita-Calvo C, Vázquez M (2017) Differences between honeydew and blossom honeys: a review. Trends Food Sci Technol 59:79–87. https://doi.org/10.1016/j.tifs.2016.11.015
Acknowledgements
Thanks to Merck, Darmstadt, Germany, for support regarding plate material
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors, WS and GM are members of the Editorial Board of the journal. Therefore, the submission was handled by a different member of the editorial board, and they did not take part in the review process in any capacity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwack, W., Heilmann, D. & Morlock, G.E. Ethylamine as new derivatization reagent differentiating reducing from non-reducing saccharides. JPC-J Planar Chromat 36, 359–366 (2023). https://doi.org/10.1007/s00764-023-00266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-023-00266-9