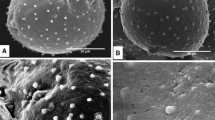
Abstract
Variations in pollen characters and tapetum behavior were recently acknowledged in the early-divergent family Nymphaeaceae and even within the genus Nymphaea, which probably is not monophyletic; some traits such as infratectum and tapetum type are also a matter of different interpretations. In this study, developmental characters of the pollen grains and tapetum in Nymphaea subgenus Hydrocallis are provided for the first time. Observations were made in N. amazonum, N. gardneriana, and N. prolifera using light, scanning, and transmission electron microscopy. Tapetum is of the secretory type and produces orbicules. At microspore and pollen grain stages, the distal and proximal walls differ considerably. This result supports the operculate condition of the aperture in Hydrocallis, and such aperture might be plesiomorphic for Nymphaeoideae. The infratectum is intermediate, composed of inter-columellae granular elements, robust columellae consisting of agglomerated granules, complete columellae, and fused columellae. Narrow microchannels are present and persist until the mature pollen grain stage. The membranous granular layer is often present in the pollen grains of Nymphaeaceae. In N. gardneriana, this layer is most probably a component of the intine because it is lost after acetolysis. Orbicules in the Nymphaeaceae are characterized as spherical or subspherical, with a smooth sporopolleninic wall that surrounds an electron-lucent core and with individual orbicules that usually merge to give irregular aggregations. The aperture, pollen wall ultrastructure, and the tapetum of the studied species are discussed in an evolutionary and systematic context, and these characters are also compared with those of other angiosperm lineages.







Similar content being viewed by others
References
Ansari R, Jeeja G, Jayalakshmi SK (2005) Pollen morphology of Nymphaea Linn. J Palynol 41:139–152
APG IV (2016) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181:1–20
Bhowmik S, Datta BK (2012) Pollen dimorphism of several members of Nymphaeaceae and Nelumbonaceae: an index of geographical and ecological variation. Not Sci Biol4:38–44
Borsch T, Hilu KW, Wiersema JH, Löhne C, Barthlott W, Wilde V (2007) Phylogeny of Nymphaea (Nymphaeaceae): evidence from substitutions and microstructural changes of the chloroplast trnT-F region. Int J Plant Sci 168:639–671
Borsch T, Löhne C, Wiersema JW (2008) Phylogeny and evolutionary patterns in Nymphaeales: integrating genes, genomes and morphology. Taxon 57:1052–1081
Borsch T, Löhne C, Mbaye MS, Wiersema J (2011) Towards a complete species tree of Nymphaea: shedding further light on subgen. Brachyceras and its relation-ships to the Australian water-lilies. Telopea 13:193–217
Chapman GP (1987) The tapetum. Int Rev Cytol 107:111–125
Coiro M, Barone Lumaga MR (2013) Aperture evolution in Nymphaeaceae: insights from a micromorphological and ultrastructural investigation. Grana 52:192–201
Dai H, Zhou Q (2010) Embryological studies in Nymphaea lotus (Nymphaeaceae). Acta Bot Boreali-Occidentalia Sinica 30:78–84 (in Chinese)
Debasis B, Mondal AK (2012) Studies on production, morphology and free amino acids of pollen of four members in the genus Nymphaea L. (Nymphaeaceae). Int J Sci Nat 3:705–718
Doyle JA (2005) Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 44:227–251
Doyle JA (2009) Evolutionary significance of granular exine structure in the light of phylogenetic analyses. Rev Palaeobot Palynol 156:198–210
Doyle JA, Endress PK (2000) Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int J Plant Sci 161(S6):S121–S153
Echlin P, Godwin H (1969) The ultrastructure and ontogeny of pollen in Helleborus foetidus L. III. The formation of the pollen grain wall. J Cell Sci 5:459–477
El-Ghazaly G, Huysmans S (2001) Re-evaluation of a neglected layer in pollen wall development with comments on its evolution. Grana 40:3–16
El-Ghazaly G, Huysmans S, Smets EF (2001) Pollen development of Rondeletia odorata (Rubiaceae). Am J Bot 88:14–30
Endress PK, Doyle JA (2009) Reconstructing the ancestral angiosperm flower and its initial specializations. Am J Bot 96:22–66
Erdtman GB (1960) The acetolysis method: a revised description. Svensk Bot Tidskr 54:561–564
Erdtman GB, Berglund B, Praglowski J (1961) An introdution to a Scandinavian pollen flora. Alnqvist & Wiksell, Uppsala
Furness CA (2008) A review of the distribution of plasmodial and invasive tapeta in Eudicots. Int J Plant Sci169:207–223
Furness CA, Rudall PJ (2001) The tapetum in basal angiosperms: early diversity. Int J Plant Sci 162:375–392
Furness CA, Rudall PJ (2003) Apertures with lids: distribution and significance of operculate pollen in monocotyledons. Int J Plant Sci164:835–854
Gabarayeva NI (1991) Patterns of development in primitive angiosperm pollen. In: Blackmore S, Barnes SH (eds) Pollen and spores: patterns of diversification. Calderon Press, Oxford, pp 257–268
Gabarayeva NI (1996) Sporoderm development in Liriodendron chinense (Magnoliaceae): a probable role of the endoplasmic reticulum. Nord J Bot 16:307–323
Gabarayeva NI, El-Ghazaly G (1997) Sporoderm development in Nymphaea mexicana (Nymphaeaceae). Plant Syst Evol 204:1–19
Gabarayeva NI, Grigorjeva VV (2010) Sporoderm ontogeny in Chamaedorea microspadix (Arecaceae): self-assembly as the underlying cause of development. Grana 49:91–114
Gabarayeva NI, Grigorjeva VV (2012) Sporoderm development and substructure in Magnolia sieboldii and other Magnoliaceae: an interpretation. Grana 51:119–147
Gabarayeva NI, Hemsley AR (2006) Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139
Gabarayeva NI, Rowley JR (1994) Exine development in Nymphaea colorata (Nymphaeaceae). Nord J Bot 14:671–691
Gabarayeva NI, Walles B, El-Ghazaly G, Rowley JR (2001) Exine and tapetum development in Nymphaea capensis (Nymphaeaceae): a comparative study. Nord J Bot 21:529–548
Gabarayeva NI, Grigorjeva VV, Rowley JR (2003) Sporoderm ontogeny in Cabomba aquatica (Cabombaceae). Rev Palaeobot Palynol 127:147–173
Gabarayeva NI, Grigorjeva VV, Polevova S, Hemsley AR (2016) Pollen wall and tapetum development in Plantago major L. (Plantaginaceae): assisting self-assembly. Grana. doi:10.1080/00173134.2016.1159729
Galati BG (1985) Estudios embriológicos en Cabomba australis (Nymphaeaceae) I. La esporogénesis y las generaciones sexuadas. Bol Soc Argent Bot 24:9–47
Galati BG (2003) Ubisch bodies in Angiosperms. In: Pandey AK, Dhakal MR (eds) Advances in plant reproductive biology, vol II. Narendra, Delhi, pp 1–21
Galati BG, Zarlavsky G, Rosenfeldt S, Gotelli MM (2012) Pollen ontogeny in Magnolia liliflora Desr. Plant Syst Evol 298:527–534
Gonzalez AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 9:287–294
Grigorjeva V, Gabarayeva N (2015) The development of sporoderm, tapetum and Ubisch bodies in Dianthus deltoides (Caryophyllaceae): self-assembly in action. Rev Palaeobot Palynol 219:1–27
Hesse M, Zetter R (2005) Ultrastructure and diversity of recent and fossil zona-aperturate pollen grains. Plant Syst Evol 255:145–176
Ito M (1984) Studies in the floral morphology and anatomy of Nymphaeales. II. Floral anatomy of Nymphaea tetragona George. Acta Phytotax Geobot 35:94–102
Ito M (1987) Phylogenetic systematics of the Nymphaeales. Bot Mag (Tokyo) 100:17–35
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Jones MR, Clarke GCS (1981) Nymphaeaceae. Rev Palaeobot Palynol 33:57–67
Khanna P (1964) Morphological and embryological studies in Nymphaeaceae I: Euryale ferox Salisb. Proc Indian Acad Sci 59:237–243
Khanna P (1965) Morphological and embryological studies in Nymphaeaceae. II: Brasenia schreberi Gmel. and Nelumbo nucifera Garten. Aust J Bot 13:379–387
Khanna P (1967) Morphological and embryological studies in Nymphaeaceae III. Victoria cruziana D'Orb. and Nymphaea stellata Willd. Bot Mag (Tokyo) 80:305–312
Kreunen SS, Osborn JM (1999) Pollen and anther development in Nelumbo (Nelumbonaceae). Am J Bot 86:1662–1676
Les DH, Schneider EL, Padgett DJ, Soltis PS, Soltis DE, Zanis M (1999) Phylogeny, classification and floral evolution of water lilies (Nymphaeaceae; Nymphaeales): a synthesis of non-molecular, rbcL, matK, and 18S rDNA data. Syst Bot 24:28–46
Löhne C, Borsch T, Wiersema JH (2007) Phylogenetic analysis of Nymphaealesusing fast-envolving and noncoding chloroplast markers. Bot J Linn Soc 154:141–163
Lu L, Wortley AH, Li D, Wang H, Blackmore S (2015) Evolution of angiosperm pollen. 2. The basal angiosperms. Ann Mo Bot Gard 100:227–269
Luque R, Sousa HC, Graus JE (1996) Métodos de coloracão de Roeser (1972) modificado Kropp, E., 1972. Visando a substituicão do azul de astra por azul de alcião8GS ou 8GX. Acta Bot Brasil 10:199–212
Meyer NR (1964) Palynological studies in Nymphaeaceae. Bot Zhurn 49:1421–1429
Meyer-Melikian NR, Diamondopulu N (1996) Ultrastructure of pollen grains of the order Nymphaeales. Bot Zhurn 81:1–9 (in Russian)
Murthy GVS (2000) Pollen morphology of Nymphaeaceae (s.L.). Bull Bot Surv India 42:73–80
Osborn JM (2000) Pollen morphology and ultrastructure of gymnospermous anthophytes. In: Harley MM, Morton CM, Blackmore S (eds) Pollen and spores: morphology and biology pp 163–185
Osborn JM, Taylor TN, Schneider EL (1991) Pollen morphology and ultrastructure of the Cabombaceae: correlations with pollination biology. Am J Bot 78:1367–1378
Pacini E (1990) Tapetum and microspore function. In: Blackmore S, Knox RB (eds) Microspores, evolution and ontogeny. Academic Press, London, pp 213–237
Pacini E (1997) Tapetum character states: analytical keys for tapetum types and activities. Can J Bot 75:1448–1459
Pacini E, Franchi GG (1993) Role of the tapetum in pollen and spore dispersal. Plant Syst Evol 7:1–11
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Papini A, Mosti S, van Doorn WG (2013) Classical macroautophagy in Lobivia rauschii (Cactaceae) and possible plastidial autophagy in Tillandsia albida (Bromeliaceae) tapetum cells. Protoplasma 251:719–725. doi:10.1007/s00709-013-0567-y
Podoplelova Y, Ryzhakov G (2005) Phylogenetic analysis of the order Nymphaeales based on the nucleotide sequences of the chloroplast ITS2–4 region. Plant Sci 169:606–611
Remizowa MV, Sokoloff DD, Macfarlane TD, Yadav SR, Prychid CJ, Rudall PJ (2008) Comparative pollen morphology in the early-divergent angiosperm family Hydatellaceae reveals variation at the infraspecific level. Grana 47:81–100
Roland F (1965) I'rkisions sur la structure el I'ultrastructure d'unc tktradc calymmee. Pollen Spores 7:5–8
Rowley JR (1993) Cycles of hyperactivity in tapetal cells. In: Hesse M, Pacini E, Willemse M (eds) The tapetum, cytology, function, biochemistry, and evolution. Springer, Vienna, pp 23–37
Rowley JR, El-Ghazaly G, Rowley JS (1987) Microchannels in the pollen grain exine. Palynology 11:1–21
Rowley JR, Gabarayeva NI, Walles B (1992) Cyclic invasion of tapetal cells into loculi during microspore development in Nymphaea colorata (Nymphaceae). Am J Bot 79:801–808
Rowley JR, Skvarla JJ, El-Ghazaly G (2003) Transfer of material through the microspore exine from the loculus into the cytoplasm. Can J Bot 81:1070–1082
Sampson FB (2007) Variation and similarities in pollen features in some basal angiosperms, with some taxonomic implications. Plant Syst Evol 263:59–75
Sharma A, Singh MB, Bhalla PL (2015) Anther ontogeny in Brachypodium distachyon. Protoplasma 252:439–450
Singh CB, Motial VS, Nair PKK (1969) Pollen morphology of Nymphaea. Plant Sci 1:53–56
Skvarla JJ, Larson DA (1966) Fine structural studies of Zea mays pollen I: cell membranes and exine ontogeny. Am J Bot 53:1112–1125
Taylor ML, Osborn JM (2006) Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Am J Bot 93:344–356
Taylor ML, Gutman BL, Melrose NA, Ingraham AM, Schwartz JA, Osborn JM (2008) Pollen and anther ontogeny in Cabomba caroliniana (Cabombaceae, Nymphaeales). Am J Bot 95:399–413
Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Schwartz GJ, Thiemann TC, Osborn JM (2012) Tapetum structure and ontogeny in Victoria (Nymphaeaceae). Grana 51:107–118
Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Schwartz GJ, Thiemann TC, Osborn JM (2013) Pollen ontogeny in Victoria (Nymphaeales). Int J Plant Sci 174:1259–1276
Taylor ML, Cooper RL, Schneider EL, Osborn JM (2015) Pollen structure and development in Nymphaeales: insights into character evolution in an ancient angiosperm lineage. Am J Bot 102:1685–1702
Tsou CH, Cheng PC, Tseng CM, Yen HJ, Fu YL, You TR, Walden DB (2015) Anther development of maize (Zea mays) and longstamen rice (Oryza longistaminata) revealed by cryo-SEM, with foci on locular dehydration and pollen arrangement. Plant Reprod 28:47–60
Volkova PA, Shipunov AB (2008) Morphological variation of Nymphaea (Nymphaeaceae) in European Russia. Nord J Bot 25:329–338
Walker JW (1974a) Evolution of exine structure in the pollen of primitive angiosperms. Am J Bot 61:891–902
Walker JW (1974b) Aperture evolution in the pollen of primitive angiosperms. Am J Bot 61:1112–1137
Walker JW, Doyle JA (1975) The bases of angiosperm phylogeny: palynology. Ann Mo Bot Gard 62:664–723
Weber M, Ulrich S (2010) The endexine: a frequently overlooked pollen wall layer and a simple method for detection. Grana 49:83–90
Wiersema JH (1987) A monograph of Nymphaea subgenus Hydrocallis (Nymphaeaceae). Syst Bot Monogr 16:1–112
Wodehouse RP (1935) Pollen grains. Their structure, identification and significance in science and medicine. McGraw-Hill, New York
Xu FU, Kirchoff BK (2008) Pollen morphology and ultrastructure of selected species of Magnoliaceae. Rev Palaeobot Palynol 150:140–153
Zarlavsky GE (2014) Histología Vegetal. Técnicas simples y complejas. Soc Arg Bot. Buenos Aires, Argentina
Zavada MS (1984) Pollen wall development of Austrobaileya maculata. Bot Gaz 145:11–21
Zhou Q, Fu D (2008) Reproductive morphology of Nuphar (Nymphaeaceae), a member of basal angiosperms. Plant Syst Evol 272:79–96
Acknowledgements
Financial support for this research was provided by the Universidad de Buenos Aires (UBACyT 20020120100056BA), Agencia Nacional de Promoción Científica, Tecnológica y de Innovación, Argentina (ANPCyT-UNNE, PICTO 2012-0202), and the Universidad Nacional del Nordeste (PI A012-2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Alexander Schulz
Rights and permissions
About this article
Cite this article
Zini, L.M., Galati, B.G., Zarlavsky, G. et al. Developmental and ultrastructural characters of the pollen grains and tapetum in species of Nymphaea subgenus Hydrocallis . Protoplasma 254, 1777–1790 (2017). https://doi.org/10.1007/s00709-016-1074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1074-8