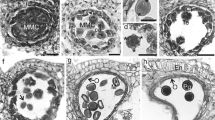
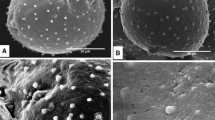
Abstract
Studies of pollen wall development produce a great deal of morphological data that supplies useful information regarding taxonomy and systematics. We present the exine development of Euptelea and Pteridophyllum, two taxa whose pollen wall development has never previously been studied using transmission electron microscopy. Both genera are representatives of the two earliest-diverging families of the order Ranunculales and their pollen data are important for the diagnosis of the ancestral pollen features in eudicots. Our observations show these genera are defined by having microechinate microreticulate exine ornamentation, perforate tectum, columellate morphology of the infratectum and the existence of a foot layer and endexine. The presence of lamellations is detected during the early stages of development in the nexine of both genera, especially in the apertures. Euptelea presents remains of the primexine layer during the whole maturation process, a very thin foot layer, and a laminate exinous oncus in the apertural region formed by ectexine and endexine elements. Pteridophyllum has a thicker tectum than Euptelea, a continuous foot layer and a thicker endexine. In the apertures, the exinous oncus is formed by islets and granules of endexine, in contrast to the Euptelea apertures. The secretory tapetum produces orbicules in both genera, but they have different morphology and electron-density. Comparisons with pollen data from related orders and families confirm the ancestral states for the pollen of eudicots proposed in previous studies: reticulate and echinate surfaces, columellate infractectum and a thin foot layer relative to the thickness of the ectexine. According to our observations, we propose considering the possibility of a polymorphic state for the aperture number in the ancestor of Ranunculales, and suggest the development of orbicules as the ancestral state in this order.





Similar content being viewed by others
References
Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Blackmore S, Stafford P, Persson V (1995) Palynology and systematics of Ranunculiflorae. Plant Syst Evol 9 (Suppl):71–82
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Blackmore S, Wortley AH, Skvarla JJ, Gabarayeva NI, Rowley JR (2010) Developmental origins of structural diversity in pollen walls of Compositae. Plant Syst Evol 284:17–32
Candau P (1987) Fumariaceae. In: Váldes B, Díez MJ, Fernández I (eds) Atlas polínico de Andalucía occidental. Instituto de desarrollo regional n 43, Univ Sevilla, Excma Diputación de Cádiz, pp 84–89
Clarke GCS, Punt W, Hoen PP (1991) Ranunculaceae. The northwest European pollen flora, 51. Rev Palaeobot Palynol 69:117–271
Claxton F, Banks H, Klitgaard BB, Crane PR (2005) Pollen morphology of families Quillajaceae and Surianaceae (Fabales). Rev Palaeobot Palynol 133:221–233
Dahl ÅE (1990) Infrageneric division of the genus Hypecoum (Papaveraceae). Nord J Bot 10:129–140
Dobritsa AA, Coerper D (2012) The novel plant protein IN APERTURATE POLLEN1 marks distinct cellular domains and control formation of apertures in the Arabidopsis pollen exine. Plant Cell 24:4452–4464
Doyle JA (2005) Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 44:227–251
Doyle JA (2009) Evolutionary significance of granular exine structure in the light of phylogenetic analyses. Rev Palaeobot Palynol 156:198–210
Echlin P, Godwin H (1969) The ultrastructure and ontogeny of the pollen in Helleborus foetidus L. III. The formation of the pollen grain wall. J Cell Sci 5:459–477
Erdtman G (1952) Pollen morphology and plant taxonomy. Almqvist and Wiksell, Stockholm
Erdtman G (1960) The acetolysis method, a revised description. Svensk Botanisk Tidskrift 54:561–564
Erdtman G (1969) Handbook of palynology. Morphology, taxonomy, ecology: an introduction to the study of pollen grains and spores. Munksgaard, Copenhagen
Fernández MC, Rodríguez-García MI (1989) Developmental changes in the aperture during pollen grain ontogeny in Olea europaea L. New Phytol 111:717–723
Furness CA (2008a) Successive microsporogenesis in eudicots, with particular reference to Berberidaceae (Ranunculales). Plant Syst Evol 273:211–223
Furness CA (2008b) A review of the distribution of plasmodial and invasive tapeta in eudicots. Int J Plant Sci 169:207–223
Furness CA, Rudall PJ (2001) The tapetum in basal angiosperms: early diversity. Int J Plant Sci 162:357–392
Furness CA, Magallón S, Rudall PJ (2007) Evolution of endoapertures in early-divergent eudicots, with particular reference to pollen morphology in Sabiaceae. Plant Syst Evol 263:77–92
Gabarayeva NI, Hemsley AR (2006) Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139
Gabarayeva NI, Grigorjeva VV, Rowley JR, Hemsley AR (2009) Sporoderm development in Treversia burckii (Araliaceae) II. Post tetrad period: further evidence for the participation of self-assembly processes. Rev Palaeobot Palynol 156:233–247
Gabarayeva NI, Grigorjeva VV, Rowley JR (2010) Sporoderm development in Acer tataricum (Aceraceae): an interpretation. Protoplasma 247:65–81
Gabarayeva NI, Grigorjeva VV, Kosenko Y (2013a) I. Primexine development in Passiflora racemosa Brot.: overlooked aspects of development. Plant Syst Evol 299:1013–1035
Gabarayeva NI, Grigorjeva VV, Kosenko Y (2013b) II. Exine development in Passiflora racemosa Brot.: post-tetrad period Overlooked aspects of development. Plant Syst Evol 299:1037–1055
Hemsley AR, Gabarayeva NI (2007) Exine development: the importance of looking through a colloid chemistry window. Plant Syst Evol 263:25–49
Hesse M, Halbritter H, Zetter R, Weber R, Buchner R, Frosh-Radivo A, Ullrich S (2009) Pollen terminology: an illustrated handbook. Springer, New York
Hoot SB, Kadereit JW, Blattner FR, Jork KB, Schwarzbach AE, Crane PR (1997) Data congruence and phylogeny of the Papaveraceae sl based on four data sets: atpB and rbcL sequences, trnK restriction sites, and morphological characters. Syst Bot 22:575–590
Hoot SB, Magallón-Puebla S, Crane PR (1999) Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL and 18S nuclear ribosomal DNA sequences. Ann Missouri Bot Gard 86:119–131
Hoot SB, Wefferling KM, Wulf JA (2015) Phylogeny and Character Evolution of Papaveraceae sl (Ranunculales). Syst Bot 40:474–488
Kalis AJ (1979) Papaveraceae. The Northwest European Pollen Flora 20. Rev Palaeobot Palynol 28:200–260
Kreunen SS, Osborn JM (1999) Pollen and anther development in Nelumbo (Nelumbonaceae). Am J Bot 86:1662–1676
Layka S (1976) Les méthodes modernes de la palynologie appliquées à l’étude des papaverales. Thèse d’Etat, Montpellier, p 318
Lidén M (1993) Fumariaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol. 2: flowering plants, dicotyledons: Magnoliid, hamamelid, and caryophyllid families. Springer, Heidelberg
Nowicke JW, Skvarla JJ (1982) Pollen morphology and the relationships of Circaeaster, of Kingdonia, and of Sargentodoxa to the Ranunculales. Am J Bot 69:990–998
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. Ecotype Wassilewskija (Brassicaceae). Protoplasma 185:7–21
Pérez-Gutiérrez MA, Suárez-Santiago VN, Fernández MC, Salinas Bonillo MJ, Romero-García AT (2015a) Pollen morphology and post-tetrad wall development in the subfamily Fumarioideae (Papaveraceae). Rev Palaeobot Palynol 222:33–47
Pérez-Gutiérrez MA, Romero-García AT, Fernández MC, Blanca G, Salinas-Bonillo MJ, Suárez-Santiago VN (2015b) Evolutionary history of fumitories (subfamily Fumariodieae, Papaveraceae): an old history shaped by the main geological and climatic events in the Northern Hemisphere. Mol Phylogenet Evol 88:75–92
Praglowski J (1974) World pollen and spore flora 3, Angiospermae. Magnoliaceae Juss, Stockholm
Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 143:1–81
Ren Y, Li H-F, Zhao L, Endress PK (2007) Floral morphogenesis in Euptelea (Eupteleaceae, Ranunculales). Ann Bot 100:185–193
Romero AT, Fernández MC (2000) Development of exine and apertures in Fumaria densiflora DC from the tetrad stage to maturity. In: Harley MM, Morton CM, Blackmore S (eds) Pollen and spores: Morphology and Biology. Royal Botanic Gardens, Kew, pp 45–56
Romero AT, Salinas MJ, Fernández MC (2003) Pollen wall development in Hypecoum imberbe (Fumariaceae). Grana 42:91–101
Rowley JR (1992) Pollen of Cercidiphyllum (Cercidiphyllaceae). Bot Zh Ross Akad Nauk 77:1–3
Sauquet H, Carrive L, Poullain N, Sannier J, Damerval C, Nadot S (2015) Zygomorphy evolved from disymmetry in Fumarioideae (Papaveraceae, Ranunculales): new evidence from an expanded molecular phylogenetic framework. Ann Bot 115:895–914
Soltis DE, Smith SA, Cellinese N et al (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot 98:704–730
Taylor ML, Cooper RL, Schneider EL, Osborn JM (2015) Pollen structure and development in Nymphaeales: Insights into character evolution in an ancient angiosperm lineage. Am J Bot 102:1685–1702
Teixeira MDR, Amorim AM, Riberio FA (2013) Pollen morphology of Menispermaceae in the state of Bahia, Brazil. Acta Bot Bras 27:436–444
Van Campo M (1971) Précisions nouvelles sur les structures comparées des pollens de Gymnospermes et d’Angiospermes. CompteRendudel’Académiedes Sciencesde France 272:2071–2074
Verstraete B, Moon H-K, Smets E, Huysmans S (2014) Orbicules in flowering plants: a phylogenetic perspective on their form and function. Bot Rev 80:107–134
Walker JW (1974) Aperture evolution in the pollen grains of primitive angiosperms. Am J Bot 61:1112–1136
Wang W, Lu AM, Ren Y, Endress ME, Chen ZD (2009) Phylogeny and classification of Ranunculales: evidence from four molecular loci and morphological data. Perspect Plant Ecol Evol Syst 11:81–110
Worberg A, Quandt D, Barniske A-M, Löhne C, Hilu KW, Borsch T (2007) Phylogeny of basal eudicots: insights from non-coding and rapidly evolving DNA. Org Divers Evol 7:55–77
Wortley AH, Wang H, Lu L, Li D-Z, Blackmore S (2015) Evolution of angiosperm pollen. 1 Introduction. Ann Missouri Bot Gard 100:177–226
Zavada MS, Dilcher DL (1986) Comparative pollen morphology and its relationship to phylogeny of pollen in the Hamamelidae. Ann Missouri Bot Gard 73:348–381
Acknowledgments
This work was funded by the Spanish Ministry of Science and Innovation (CGL2008-01554/BOS) and by the Andalusian Regional Ministry of Economy, Innovation and Science (P12.RNM2680). We thank the staff of the National Botanic Garden of Belgium and of the Gothenburg Botanical Garden for their support and help for sampling the pollen material, and to Concepción Hernández Castillo, María José Martínez Guerrero and Isabel Sánchez Almazo of the Scientific Instrumentation Centre of the University of Granada for preparing our samples for TEM and SEM visualisation. The authors also thank Dr. James Doyle for his helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pérez-Gutiérrez, M.A., Fernández, M., Salinas-Bonillo, M.J. et al. Comparative exine development from the post-tetrad stage in the early-divergent lineages of Ranunculales: the genera Euptelea and Pteridophyllum . J Plant Res 129, 1085–1096 (2016). https://doi.org/10.1007/s10265-016-0862-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0862-8