Abstract
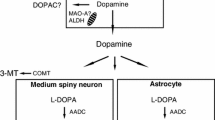
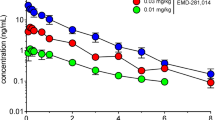
MRZ-9547 (d-(2-(2-oxo-4(R)-phenylpyrrolidin-1-yl)-acetamide) is a drug acting at the dopamine transporter (DAT). In the present study, effects of MRZ-9547 alone and in combination with L-3,4-dihydroxyphenylalanine (L-DOPA) were investigated in rodent models predictive for efficacy in Parkinson’s disease (PD) and L-DOPA-induced dyskinesia (LID). In rats pre-treated with haloperidol (0.2 mg/kg i.p.), MRZ-9547 (25–100 mg/kg i.p.) dose-dependently attenuated decrease in horizontal locomotion, activity in central zone, and rearings starting at 50 mg/kg i.p. In rats depleted of monoamines by α-methyl-p-tyrosine and reserpine treatment, MRZ-9547 attenuated hypolocomotion starting at 100 mg/kg i.p. At the doses 25–100 mg/kg i.p. the drug induced dose-dependent ipsilateral rotations in rats with unilateral 6-hydroxydopamine (6-OHDA)-induced nigrostriatal system lesions. However, MRZ-9547 enhanced contralateral rotation produced by L-DOPA given at an effective (25 mg/kg i.p.), but not at a sub-effective (6.25 mg/kg i.p.) dose. Microdialysis experiments revealed that MRZ-9547 penetrated well to the brain and did not show any pharmacokinetic interaction with L-DOPA. In unilaterally 6-OHDA-lesioned rats having developed abnormal involuntary movements (AIMs, a rodent correlate of LID) after chronic L-DOPA treatment, MRZ-9547 (50 mg/kg i.p.) did not significantly affect the AIMs expression. The results indicate that MRZ-9547 may by itself have antiparkinsonian activity at early stages of the disease, when some dopaminergic terminals are still intact. It may also enhance antiparkinsonian effect of L-DOPA. MRZ-9547 does not seem to influence the expression of LID in 6-OHDA-lesioned rats. The results support the use of MRZ-9547 in PD patients treated with L-DOPA.







Similar content being viewed by others
References
Aman MG, Singh NN (1985) Dyskinetic symptoms in profoundly retarded residents following neuroleptic withdrawal and during methylphenidate treatment. J Mental Defic Res 29(Pt 2):187–195
Balazs J, Dallos G, Kereszteny A, Czobor P, Gadoros J (2011) Methylphenidate treatment and dyskinesia in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 21(2):133–138
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618
Brooks DJ, Pavese N (2010) Imaging non-motor aspects of Parkinson’s disease. Prog Brain Res 184:205–218
Cenci MA, Lee CS, Bjorklund A (1998) L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10(8):2694–2706
Chaudhuri KR, Odin P (2010) The challenge of non-motor symptoms in Parkinson’s disease. Prog Brain Res 184:325–341
Chiueh CC, Moore KE (1975) Blockade by reserpine of methylphenidate-induced release of brain dopamine. J Pharmacol Exp Ther 193(2):559–563
Chopin P, Colpaert FC, Marien M (1999) Effects of alpha-2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway. J Pharmacol Exp Ther 288(2):798–804
Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W (2006) Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull 69:318–326
Dekundy A, Lundblad M, Danysz W, Cenci MA (2007) Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179(1):76–89
Devos D, Moreau C, Delval A, Dujardin K, Defebvre L, Bordet R (2013) Methylphenidate: a treatment for Parkinson’s disease? CNS Drugs 27(1):1–14
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164(4):1357–1391
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa-induced dyskinesias. Mov Disord 22(10):1379–1389 quiz 1523
Finn IB, Iuvone PM, Holtzman SG (1990) Depletion of catecholamines in the brain of rats differentially affects stimulation of locomotor activity by caffeine, D-amphetamine, and methylphenidate. Neuropharmacology 29(7):625–631
Friedman J, Abrantes A, Sweet LH (2011) Fatigue in Parkinson´s disease. Expert Opin Pharmacother 12:1999–2007
Harrington ME (2012) Neurobiological studies of fatigue. Prog Neurobiol 99(2):93–105
Lewitt PA (1993) Levodopa therapeutics—new treatment strategies. Neurology 43(12 Suppl. 6):31–37
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15(1):120–132
Meredith GE, Kang UJ (2006) Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord 21(10):1595–1606
Moreau C, Delval A, Defebvre L, Dujardin K, Duhamel A, Petyt G, Vuillaume I, Corvol JC, Brefel-Courbon C, Ory-Magne F, Guehl D, Eusebio A, Fraix V, Saulnier PJ, Lagha-Boukbiza O, Durif F, Faighel M, Giordana C, Drapier S, Maltete D, Tranchant C, Houeto JL, Debu B, Sablonniere B, Azulay JP, Tison F, Rascol O, Vidailhet M, Destee A, Bloem BR, Bordet R, Devos D, Parkgait II sg (2012) Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol 11(7):589–596
Nutt JG, Carter JH, Sexton GJ (2004) The dopamine transporter: importance in Parkinson’s disease. Ann Neurol 55(6):766–773
Nutt JG, Carter JH, Carlson NE (2007) Effects of methylphenidate on response to oral levodopa: a double-blind clinical trial. Arch Neurol 64(3):319–323
Paxinos G, Watson C (1986) The rats brain in stereotaxic coordinates. Academic Press, New York
Reavill C, Jenner P, Marsden CD (1983) Differentiation of dopamine agonists using drug-induced rotation in rats with unilateral or bilateral 6-hydroxydopamine destruction of ascending dopamine pathways. Biochem Pharmacol 32(5):865–870
Sayers AC, Handley SL (1973) A study of the role of catecholamines in the response to various central stimulants. Eur J Pharmacol 23(1):47–55
Smith Y, Wichmann T, Factor SA, DeLong MR (2012) Parkinson’s disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37(1):213–246
Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W (2014) The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol 25:1–12
Zhang Y, Huo M, Zhou J, Xie S (2010) PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99(3):306–314
Zvejniece L, Svalbe B, Veinberg G, Grinberga S, Vorona M, Kalvinsh I, Dambrova M (2011) Investigation into stereoselective pharmacological activity of phenotropil. Basic Clin Pharmacol Toxicol 109(5):407–412
Acknowledgments
The authors are or were at the time of experimental work employees of Merz Pharmaceuticals which was developing MRZ-9547.
Conflict of interest
No further conflicts of interest are applying.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dekundy, A., Mela, F., Hofmann, M. et al. Effects of dopamine uptake inhibitor MRZ-9547 in animal models of Parkinson’s disease. J Neural Transm 122, 809–818 (2015). https://doi.org/10.1007/s00702-014-1326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1326-8