Abstract
Purpose
In pediatric hydrocephalus (HC) treatment, programmable gravitational valves offer greater flexibility to manage overdrainage during children’s growth. However, it remains unclear whether these devices provide better outcomes rather than their precursors. The study assessed the benefit from programmability of gravitational valve, i.e., programmable-SHUNTASSISTANT (proSA®) vs. SHUNTASSISTANT® (SA®).
Methods
Clinical records and imaging of pediatric patients with hydrocephalus of non-tumoral etiology treated with fixed (SA®) or programmable (proSA®) gravitational valves between January 2006 and January 2022 were analyzed in a retrospective single-center study. Valve survival was compared in relation to age and etiology. Lately explanted valves received biomechanical analysis.
Results
A total of 391 gravitational valves (254 SA® and 137 proSA®) were inserted in 244 patients (n = 134 males). One hundred thirty-three SA® (52.4%) and 67 proSA® (48.9%) were explanted during a follow-up of 81.1 ± 46.3 months. Valve survival rate at 1 and 5 years with proSA® was 87.6% and 60.6% compared to 81.9% and 58.7% with SA®, with mean survival time 56.4 ± 35.01 and 51.4 ± 43.0 months, respectively (P = 0.245). Age < 2 years at implantation correlated with significantly lower valve survival rates (P < 0.001), while HC etiology showed no significant impact. Overdrainage alone accounted for more SA® revisions (39.8% vs. 3.1%, P < 0.001), while dysfunctions of the adjustment system represented the first cause of valve replacement in proSA® cohort (45.3%). The biomechanical analysis performed on 41 proSA® and 31 SA® showed deposits on the valve’s internal surface in 97.6% and 90.3% of cases.
Conclusion
Our comparative study between proSA® and SA® valves in pediatric HC demonstrated that both valves showed similar survival rates, regardless of etiology but only with young age at implantation. The programmability may be beneficial in preventing sequelae of chronic overdrainage but does not reduce need for valve revision and proSA® valve should be considered in selected cases in growing children older than 2 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventriculoperitoneal (VP) shunting is a common treatment for hydrocephalus (HC) in children [15]. However, associated complications can result in frequent revisions, impacting a child’s quality of life and development [7, 13, 14]. Chronic overdrainage of cerebrospinal fluid (CSF) after VP shunt is a challenging complication that can contribute to clinical deterioration [7, 13, 22, 23, 29], and the economic burden of repeated valve replacements [26]. A “siphon effect” created by gravity and hydrostatic force when the patient is upright causes overdrainage [10]. This can lead to postural headaches, as well as subdural hematoma/hygromas, slit-ventricle syndrome, and secondary craniosynostosis. It also predisposes the patient to other mechanical complications, such as ventricular catheter occlusion [7, 20].
The primary cause of overdrainage after VP shunt implantation is the effect of posture [20], and numerous overdrainage-preventing devices have been developed on that principle [14]. Gravitational valves (e.g., Miethke SHUNTASSISTANT, SA®) are antisiphons implanted in tandem with standard differential pressure (DP) valves to prevent postural overdrainage. Their combination with adjustable DP valves also addresses other predisposing factors, e.g., anatomical circumstances, physical activity, and CSF imbalance [9]. Throughout the process of growth, alterations in these factors can lead to the progression of excessive drainage even after adjustments are made to the DP valve. As a result, it may be necessary to implant a new gravitational valve that has a higher pressure threshold to address the issue [8, 14]. The introduction of more advanced programmable gravitational valves (e.g., Miethke programmable-SHUNTASSISTANT, proSA®) enabled the non-invasive tuning of the pressure settings, improving the compliance of the antisiphon device. Nonetheless, the benefits of programmability may be undermined by new mechanical complications, such as blockage of the valve adjustment system by protein deposits [17], and unintentional changes in the pressure setting. Therefore, selecting the most suitable device for pediatric HC patients is crucial to manage overdrainage effectively and minimize the need for additional revisions.
We aimed to evaluate the advantages of utilizing programmable gravitational valves (proSA® versus SA®) in our pediatric patients and to identify the risk factors that contribute to frequent valve dysfunctions, taking into account etiological and age-related differences. Gaining a deeper understanding of these risk factors would permit a more personalized approach to the use of these valves, resulting in a more favorable cost-benefit ratio while reducing the need for additional valve revisions and associated risks to the patients.
Methods
Study design and patient sample
In a retrospective single-center study, we assessed efficacy of fixed (SA®) and programmable (proSA®) gravitational valves in treating pediatric hydrocephalus patients. We included all patients harboring ventriculoperitoneal or ventriculoatrial shunts with a minimum follow-up of 12 months after implantation between January 2006 and January 2022. All parents or patients’ guardians signed informed consent for participation in that study. The institutional ethics committee approved the study (S-084/2022); we excluded patients with tumor-related hydrocephalus as their survival depends on oncological disease and they are more prone to valve dysfunction due to possible presence of tumor cells in their CSF. Primary outcome was to compare survival rates of the two valves using Kaplan-Meier survival analysis and number of shunt revisions. Secondary objective was to identify potential risk factors for valve dysfunction by subgroup analysis according to etiology (posthemorrhagic, aqueduct stenosis, and Chiari malformation type II) and age (0–2, 2–10, and 10–18 years) groups, facilitating informed decisions when selecting valves for the treatment of pediatric hydrocephalus patients. Since 2017, all patients under the age of 6 months and all those who need reoperation on their shunt due to CSF infection received an antibacterial impregnated catheter. Before 2017, the indication for such catheters was only made individually.
Infection in our study included CSF, wound, and peritoneal infections. Each infection was determined individually according to established criteria. CSF was examined in the case of CSF infection, microbiological material was examined in the case of wound infection, and microbiological material was surgically obtained in the case of abdominal infection. For all types of infection, proof of pathogens was required.
Clinical and radiological parameters
Data about patients’ demographics, HC etiology, shunt/valve insertion indication, pre-/postoperative clinical and radiological assessments, frequency of pressure settings adjustments, and shunt revisions were collected from our central database. Valves explanted starting from mid-2017 were sent to the manufacturer’s laboratory (MIETHKE) for biomechanical analysis. Pederesen et al. defined overdrainage as the persistency of clinical symptoms and signs as postural dependent headache and vomiting, sunken fontanelle, decreasing head circumference and/or radiological signs (slit ventricles and/or subdural collections) [21]. Good clinical outcome was defined as stable improvement of the preoperative symptoms within the first postoperative year, not requiring valve explantation. The evaluation of the postoperative correction of chronic signs of overdrainage, such as arrested head growth, was conducted during both short-term and long-term follow-up periods. The objective was to ensure continued head growth aligned with the initial percentile curve. Patients with preoperative slit-ventricle morphology were evaluated to determine the radiological outcome. A normalized ventricular size following surgery was considered an improved radiological finding.
Biochemical analysis of explanted valves
The biomechanical analysis assessed macroscopic changes or deformations in the valve casing, valve permeability, the flow resistance in cm H2O during horizontal and vertical positioning of the device, adjustability in vitro of the proSA® valves, and the presence of internal deposits. For proSA® valves, we classified the internal deposits into three grades according to their extension on the valve’s inner surface: grade 1 (< 25%), grade 2 (25–50%), and grade 3 (> 50%). Two observers rated each valve separately, with coincidental results.
Data analysis
Values were reported as mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. For intergroup comparisons, t-test was utilized to compute differences between continuous variables, and Mann-Whitney test and Fisher’s exact test for categorical variables. A P-value less than 0.05 indicated statistical significance. IBM SPSS Statistics 28 software was used for graph design and statistical analysis.
Results
Patients’ population
Three-hundred ninty-one gravitational valves (254 SA®, 137 proSA®) inserted in 244 pediatric patients (134 males) were included in the study. Figure 1 shows the inclusion and exclusion process in detail. The mean age at the time of valve insertion was 5.51 ± 5.15 years in the proSA® cohort and 5.8 ± 5.52 in the SA® cohort (P = 0.608), while the mean time of follow-up was 78.19 ± 30.19 and 82.70 ± 52.96 months, respectively (P = 0.284). (See Table 1.)
Early wound complications
After valve implantation, within the first month, a total of eighteen valves (4.6%) were replaced due to wound infections and healing disorders. Among these replaced valves, 3 were proSA® and 15 were SA® valves. Notably, all these implantations were performed in infants, with a median age of 0.27 years and a range of 0.0 to 1.0 years, exposing them to a higher risk of wound complications. None of those valves underwent biomechanical analysis by the manufacturer.
It is worth mentioning that only 38.9% of the explanted valves were as primary implants.
Valve data and revisions
The valves were inserted as first implantations in 19% and 24% of cases. The secondary implanted proSA® and SA® replaced a previous gravitational valve in 70.0% and 64.5% of the cases and a non-gravitational device in 30.0% and 35.5%. Among these cases, the indication to shunt revision was mainly given by the insufficient control of overdrainage symptoms and signs with the previous shunt system (29.4%), thus requiring the implantation or substitution of a gravitational valve with a higher opening pressure. A further frequent indication was represented by the defective pressure adjustments of the previous system (30.7%), consisting of impossible or repeated accidental adjustments with inadequate CSF control. Hence, the system was replaced to address patients’ over- or underdrainage-related symptoms/signs and prevent possible complications.
During follow-up and after the first month of implantation, 168 and 246 shunt revisions were performed in proSA® and SA® cohorts. Sixty-four proSA® (47.8%) and 118 SA® (49.4%) valves were explanted during the analyzed period (P = 0.849). In proSA® cohort, dysfunctions of the adjustment system represented the first cause of valve replacement (45.3%), followed by underdrainage (18.8%). Valve explantations related to adjustment system dysfunction were performed either to prevent or to treat the onset of over- and underdrainage manifestations resulting from this defect. Overdrainage alone without adjustment system dysfunction accounted for only 3.1% of proSA® valve revisions, while it represented the most common cause of valve replacement in the SA® cohort (41.5%). Indications for valve explantations were significantly different among the two groups (P < 0.001). (For more details, see Table 1.)
Comparative survival analysis
Valve survival rates at 1 and 5 years with proSA® were 87.6% and 60.6% compared to 81.9% and 58.7% with SA®, while the mean survival time was 56.4 ± 35.01 and 51.4 ± 43.0 months, respectively. No statistically significant difference was found in the overall valve survival rate between the two groups (P = 0.245). Inter- and intra-cohort Kaplan-Meier valve survival curves are shown in Figs. 2 and 3, respectively. Explanted and not explanted valves are compared in Table 2. Table 3 reports valve survival rates per year.
Intercohort analysis with Kaplan-Meier survival curves comparing proSA® and SA® valves. Curve A compares the entire proSA® and SA® cohorts while B, C, D, and E, F, and G compare the two cohorts under etiological and age subgroups, respectively. Etiological subgroups include posthemorrhagic (A), idiopathic aqueductal stenosis (B), and Chiari malformation type II (C). Age subgroups include 0–2 (E), 2–10 (F), and 10–18 (G) years
Regarding the etiology of hydrocephalus (posthemorrhagic HC, idiopathic aqueduct stenosis, Chiari malformation type II), both cohorts showed similar results in terms of survival time and the number of explanted vs. non explanted SA® (P = 0.057, P = 0.073) or proSA® valves (P = 0.724, P = 0.472). No significant difference in valve survival rate was observed after the inter-cohort comparison for the same etiological subgroups (P = 0.911; P = 0.686; P = 0.686).
In both cohorts, the mean patient’s age was significantly younger among the explanted valves (P < 0.01), and the valve survival rate was considerably lower in the first age subgroup (0–2 years) compared to the other two groups (2–10 and 10–18 years) (P < 0.01). Below 2 years of age, mean valve survival time was 40.5 ± 32.4 and 40.9 ± 42.1 months for proSA® and SA®, compared to 69.3 ± 39.2 and 56.7 ± 39.7 in the second group (2–10 years). We observed no significance when comparing proSA® and SA® survival rates for the three age subgroups (P = 0.954; P = 0.059; P = 0.806).
In the SA® cohort, primary implants were associated with significantly more valve explantation and a lower survival rate than the secondary implanted valves (P < 0.001). There was no significant difference in valve survival rate among proSA® and SA® for primary (P = 0.769) or secondary implants (P = 0.208). Age at implantation was significantly younger for primary than secondary implants (1.9 ± 3.8 vs. 6.8 ± 5.3 years; P < 0.001). One-year survival rate in primary inserted proSA cohort was 73.1% compared to 75.4% in primary inserted SA cohort. Additionaly, the 5-year survival rate was 42.3% in same proSA cohort vs 39.3% in SA cohort.
Biomechanical analysis
Manufacturer’s reports of the biomechanical analysis were available for 41 proSA® and 31 SA® explanted valves. Impossible (n = 21) or spontaneous adjustments (n = 3) of the pressure settings represented the first cause of valve explantation in the analyzed proSA® valves (58.4%), while overdrainage (35.5%) and suspected SA® dysfunction associated with defective DP valves (38.7%) led to significantly more valve explantation in the other group (P < 0.001). The external housing was deformed in 51.2% of the proSA® valves, while presented only scratches in 4 proSA® and 2 SA® valves. Reduced permeability or complete occlusion was documented in 46.3% and 32.3% of the proSA® and SA® valves, respectively (P = 0.241). The measurement of the flow resistance performed showed an accelerated flow after the vertical valve orientation in 50.0% of proSA® and 29.2% of SA® valves (P = 0.949). Internal deposits were observed in 97.6% and 90.3% of the proSA® and SA® valves. The proSA® internal surface was covered mainly by grade 3 (51.2%) and grade 2 (26.8%) deposits, while grade 1 extents were observed in 19.5% of the cases. The intraoperative CSF examination was utterly unremarkable in both cohorts. (For more details, see Table 4 and Fig. 4.)
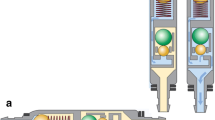
Internal deposition and deformation of the external valve housing. Figure A illustrates a severe deformation of the proSA®, while grade I, II, and III deposits can be observed in figures B, C, and D, respectively. Yellow lines divide the valves into quadrants to evaluate the deposit grade (I < 25%, II 25–50%, III > 50%). Red arrows indicate the presence of deposits in the quadrants
Clinical and radiological outcomes
In our study, pre- and postoperative clinical assessments were conducted for all implanted valves. The most common clinical manifestations were headache, nausea, increased head circumference, and arrests of head growth. A postoperative improvement was observed for 92.2% and 88.4% of the symptoms and signs in the proSA® and SA® cohorts, respectively. A arrest of the head growth rate for over 3 months in children below 2 years of age was considered an adverse finding of persistent overdrainage. This condition was observed preoperatively in 13 and 11 cases in the proSA® and SA® cohorts, respectively. After surgery, 84.6% of the proSA® cases retained regular head growth rates, compared to 54.5% of the SA®.
A postoperative radiological improvement in the previously documented slit ventricles morphology was observed in 25.4% and 26.9% of the cases in proSA® and SA® cohorts, respectively. In the proSA® cohort, the presence of preoperative slit ventricle was associated with significantly less valve explantation (P = 0.037), while no significant difference was observed for the SA® valves based on preoperative radiological findings of overdrainage (P = 0.054). This means that proSA® was more efficient in achieving clinical improvement through adjustments sparing operative revisions, while SA® was more likely to require valve revision for clinical overdrainage with corresponding radiological evidence.
Discussion
Summary of the results
This study is the first one to compare the clinical benefits and survival rates of programmable gravitational valves with their fixed gravitational valve predecessors in pediatric patients with hydrocephalus. Previous authors have documented experiences with both valve types [2, 4, 9, 11, 16, 18, 25, 30] separately. The 1- and 5-year valve survival rates were 87.6% and 60.6% for proSA®, and 81.9% and 58.7% for SA®, respectively. Young age at implantation (< 2 years) was a risk factor for valve failure in both cohorts (P < 0.001). However, no significant difference was observed in any age or etiological subgroup. The indications for valve explantation were significantly different between the two groups (P < 0.001), with overdrainage being the main reason for SA® explantation (41.5% vs. 3.1%) and adjustment difficulties (45.3%) in the proSA® cohort (P < 0.001). Programmable valves were more effective in controlling overdrainage than their predecessors, but they were limited by an increased susceptibility to mechanical malfunctions. The etiology of hydrocephalus, CSF protein content, and the number of valve adjustments did not contribute significantly to valve explantation, according to our study.
Programmable vs. fixed gravitational valves
Gravitation-assisted adjustable DP valves (e.g., proGAV®) have been widely used in the last two decades, showing promising results in preventing chronic overdrainage in young patients [6, 11, 25, 28, 29]. The long-term valve survival rates of gravitation-assisted adjustable DP valves were evaluated by Gebert et al. [9] in 93 infants, reporting valve survival rates of 77.8% and 58.2% at 12 and 85 months, respectively. Our study assessed the gravitational valve (SA®) survival rate separately, obtaining comparable results with 1- and 7-year valve survival of 87.0% and 57.3%. Recent studies assessed the use of proSA® whether with fixed DP valves (miniNAV®) in infants [4] or in adolescent and adult patients with a complex shunt history [18, 30] and reported 12 and 24 months valve survival rates of 91% and 90%, respectively. Our study observed a 1-year valve survival rate of 89.6% for proSA®, which also aligns with the prospective multicentric PROSAIKA study (89%) [16]. Besides the current emphasis on adjustable DP valves, Sokratous et al. [27] showed positive results with 235 fixed gravitational valves (paediGAV® Miethke) implanted mainly in pediatric patients, encouraging their use before others in case of uncomplicated hydrocephalus. Therefore, selecting the optimal gravitational valve remains challenging due to the lack of comparative studies and longer follow-up for proSA® valves.
Age and etiology
Premature infants and young patients with intraventricular hemorrhage represent a vulnerable group with a higher risk for shunt failure and adverse neurodevelopmental and growth outcomes [1, 5, 19, 24, 29]. In our study, young age proved to severely affect valve survival in both cohorts (P < 0.001), while posthemorrhagic etiology of hydrocephalus showed no statistical significance. Similar results were previously reported by the prospective multicentric study of Riva-Cambrin et al. [24], observing that only age (< 6 months) and no etiology affected shunt survival. We observed a higher valve survival rate for secondary implantations in both cohorts; however, this finding might be misleading given the younger age of patients at the insertion of primary implants ( 1.9 ± 3.8 vs. 6.8 ± 5.3 years; P < 0.001).
Based on our findings, it is justifiable to use a fixed gravitational valve as the primary implant for infants and young children (below 2 years of age) as during the early months after birth, these patients typically lie down and are at a lower risk of overdrainage. Therefore, the benefits of programmable valves would be limited, and the comparable survival rate would not warrant the higher cost and the risk of further revisions. However, in older children (between 2 and 10 years), the average survival time increases significantly, by approximately twofold (103.0 ± 7.3 vs. 51.2 ± 6.9 months). Therefore, in selected cases where symptomatic patients require treatment flexibility throughout the child’s growth, substituting SA® with proSA® valves would help achieve better clinical outcome and avoid the long-term sequelae of overdrainage.
Valve adjustment and overdrainage
Pressure settings were adjusted in 67.2% of the proSA® valves, allowing better control of overdrainage, representing the main indication (74.5%). Compared to the SA® group, the proSA® valve was associated with more remarkable clinical improvement, particularly regarding overdrainage-related complications, e.g., head growth arrests. Nevertheless, in addressing slit ventricular morphology, proSA® showed similar outcomes as its precursor (25.4% vs. 26.9%). The discrepancy between clinical and radiological improvement was priorly observed [30] and attributed to the need for longer time to witness ventricular morphology changes once the optimal pressure setting for the proSA® valve is reached [13, 18, 31]. As reported by Hall et al., the persistence of slit ventricles despite satisfactory clinical improvement suggests that ventricular metrics may not be accurate parameters for evaluating overdrainage correction [12].
Biomechanical analysis
Conducting a biomechanical analysis is a practical way to thoroughly evaluate the mechanical dysfunctions that result in valve revisions [3]. Internal depositions of protein and cellular materials in titanium-based valves were previously described by Ludwig et al. [17] and were observed in 97.6% and 90.3% of the analyzed proSA® and SA® valves. In proSA® valves, they were associated with more valve occlusions (34.1% vs. 16.1%) and other mechanical defects affecting the adjustment (22.0%) and braking system (22.0%). These findings suggest that the adjustability of the gravitational valves implies an increased vulnerability to damage due to protein deposits. Severe deformation of the valve casing which was observed only in the proSA® series (39.0%, P < 0.001) is probably due to multiple trials of failed adjustments.
Limitations
The findings of this study should be interpreted within the scope of its limitations. During the long observation period, multiple surgeons performed the interventions. Given the retrospective nature of the data, the cause of shunt failure assessment may be challenging and exposed to the risk of bias. The number of cases differed significantly between the two cohorts, as the type and age of the DP valve implanted. Gravitational valves were independently tested during DP valve explantation; however, uncertain intraoperative findings may have implied their precautionary explantations, thus underestimating relative survival. Our analysis did not address the potential impact of the DP unit on the performance of the gravitational unit in a dual valve system. It is important to note that the series examined in this study primarily consisted of secondary implanted valves, resulting in an average age that is higher compared to other studies that focused on the primary use of gravitational valves in pediatric patients. Biomechanical analysis of valves was conducted for only part of the observation period; hence, the number of evaluated explants was limited. A more accurate quantitative assessment of the deposits would be needed to better define their extent.
Conclusions
The proSA® and SA® valves showed almost similar long-term results and survival rates. The programmability may be beneficial regarding better control of symptoms and should be used to address complex hydrocephalus cases with symptoms and signs of chronic overdrainage. Young age at implantation still poses the leading risk factor for valve explantation. Therefore, considering their use in selected patients after 2 years of age could increase the survival time and maximize the benefits of the more expensive programmable gravitational valves. However, due to the elevated incidence of mechanical dysfunctions, the proSA® valve did not reduce the need for shunt revisions compared to its precursor. The presence of internal deposits might affect the adjustment system representing a major limitation of these sophisticated devices.
Data availability
The data presented in this study are available on request from the corresponding author.
Code availability
Not applicable.
References
Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R (2008) Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121:e1167-1177. https://doi.org/10.1542/peds.2007-0423
Alavi S, Schulz M, Schaumann A, Schwarz K, Thomale UW (2017) Valve exchange towards an adjustable differential pressure valve with gravitational unit, clinical outcome of a single-center study. Childs Nerv Syst 33:759–765
Bettag C, von der Brelie C, Freimann FB, Thomale UW, Rohde V, Fiss I (2022) In vitro testing of explanted shunt valves in hydrocephalic patients with suspected valve malfunction. Neurosurg Rev 45:571–583. https://doi.org/10.1007/s10143-021-01564-8
Bock HC, von Philipp G, Ludwig HC (2021) An adjustable gravitational valve for initial VP-shunt treatment in hydrocephalic preterm neonates and infants below 1 year of age. Childs Nerv Syst 37:3497–3507
Boynton BR, Boynton CA, Merritt TA, Vaucher YE, James HE, Bejar RF (1989) Ventriculoperitoneal shunts in low birth weight infants with intracranial hemorrhage: neurodevelopmental outcome. Ann Rev Hydroceph 5(1987):121–122
Brunner E, Schaumann A, Pennacchietti V, Schulz M, Thomale U (2022) Retrospective single-center historical comparative study between proGAV and proGAV2. 0 for surgical revision and implant duration. Childs Nerv Syst 38:1155–1163
Faulhauer K, Schmitz P (1978) Overdrainage phenomena in shunt treated hydrocephalus. Acta Neurochir 45:89–101
Fernández Cornejo VJ, Elbabaa SK (2021) Shunt technology for infants and a lifetime. Childs Nerv Syst 37:3475–3484
Gebert A-F, Schulz M, Schwarz K, Thomale U-W (2016) Long-term survival rates of gravity-assisted, adjustable differential pressure valves in infants with hydrocephalus. J Neurosurg Pediatr 17:544–551
Gutowski P, Gölz L, Rot S, Lemcke J, Thomale U-W (2020) Gravitational shunt valves in hydrocephalus to challenge the sequelae of over-drainage. Expert Rev Med Devices 17:1155–1168
Haberl EJ, Messing-Juenger M, Schuhmann M, Eymann R, Cedzich C, Fritsch MJ, Kiefer M, Van Lindert EJ, Geyer C, Lehner M (2009) Experiences with a gravity-assisted valve in hydrocephalic children. J Neurosurg Pediatr 4:288–293
Hall BJ, Gillespie CS, Hennigan D, Bagga V, Mallucci C, Pettorini B (2021) Efficacy and safety of the Miethke programmable differential pressure valve (proGAV®2.0): a single-centre retrospective analysis. Childs Nerv Syst 37:2605–2612. https://doi.org/10.1007/s00381-021-05162-3
Hall BJ, Gillespie CS, Hennigan D, Bagga V, Mallucci C, Pettorini B (2021) Efficacy and safety of the Miethke programmable differential pressure valve (proGAV® 2.0): a single-centre retrospective analysis. Child’s Nerv Syst 37:2605–2612
Huang A-P, Kuo L-T, Lai D-M, Yang S-H, Kuo M-F (2022) Antisiphon device: a review of existing mechanisms and clinical applications to prevent overdrainage in shunted hydrocephalic patients. Biomed J 45:95–108
Kahle KT, Kulkarni AV, Limbrick DD, Warf BC (2016) Hydrocephalus in children. Lancet 387:788–799
Kehler U, Kiefer M, Eymann R, Wagner W, Tschan CA, Langer N, Rohde V, Ludwig HC, Gliemroth J, Meier U (2015) PROSAIKA: a prospective multicenter registry with the first programmable gravitational device for hydrocephalus shunting. Clin Neurol Neurosurg 137:132–136
Ludwig HC, Reitemeyer M, Bock HC, Sigler M (2020) Hydrocephalus shunt therapy: current titanium shunt valve implants obstructed by internal tissue proliferations identified as extracellular matrix membranes. Childs Nerv Syst 36:2717–2724. https://doi.org/10.1007/s00381-019-04467-8
Månsson PK, Hansen TS, Juhler M (2018) The applicability of fixed and adjustable gravitational shunt valves in two different clinical settings. Acta Neurochir 160:1415–1423
Miranda P (2010) Intraventricular hemorrhage and posthemorrhagic hydrocephalus in the preterm infant. Minerva Pediatr 62:79–89
Panagopoulos D, Strantzalis G, Gavra M, Boviatsis E, Korfias S (2022) The role of antisiphon devices in the prevention of central ventricular catheter obliteration for hydrocephalus: a 15-years institution’s experience retrospective analysis. Children 9:493
Pedersen SH, Prein TH, Ammar A, Grotenhuis A, Hamilton MG, Hansen TS, Kehler U, Rekate H, Thomale UW, Juhler M (2023) How to define CSF overdrainage: a systematic literature review. Acta Neurochir (Wien) 165:429–441. https://doi.org/10.1007/s00701-022-05469-3
Pudenz RH, Foltz EL (1991) Hydrocephalus: overdrainage by ventricular shunts. A review and recommendations. Surg Neurol 35:200–212
Rekate HL (2008) Shunt-related headaches: the slit ventricle syndromes. Childs Nerv Syst 24:423–430
Riva-Cambrin J, Kestle JR, Holubkov R, Butler J, Kulkarni AV, Drake J, Whitehead WE, Wellons JC 3rd, Shannon CN, Tamber MS, Limbrick DD Jr, Rozzelle C, Browd SR, Simon TD (2016) Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study. J Neurosurg Pediatr 17:382–390. https://doi.org/10.3171/2015.6.Peds14670
Rohde V, Haberl E-J, Ludwig H, Thomale U-W (2009) First experiences with an adjustable gravitational valve in childhood hydrocephalus. J Neurosurg Pediatr 3:90–93
Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR (2008) Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr 1:131–137
Sokratous G, Hadfield O, Van Tonder L, Hennigan D, Ellenbogen J, Pettorini B, Mallucci C (2020) Management of paediatric hydrocephalous with Miethke fixed pressure gravitational valves. The Alder Hey Children’s Hospital experience. Childs Nerv Syst 36:2021–2025
Sprung C, Schlosser H-G, Lemcke J, Meier U, Messing-Jünger M, Trost HA, Weber F, Schul C, Rohde V, Ludwig H-C (2010) The adjustable proGAV shunt: a prospective safety and reliability multicenter study. Neurosurgery 66:465–474
Thomale U-W, Gebert AF, Haberl H, Schulz M (2013) Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit (proGAV) in pediatric neurosurgery. Childs Nerv Syst 29:425–431
Tschan CA, Antes S, Huthmann A, Vulcu S, Oertel J, Wagner W (2014) Overcoming CSF overdrainage with the adjustable gravitational valve proSA. Acta Neurochir 156:767–776
Weinzierl MR, Hans F-J, Stoffel M, Oertel MF, Korinth MC (2012) Experience with a gravitational valve in the management of symptomatic overdrainage in children with shunts. J Neurosurg Pediatr 9:468–472
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, Mohammed Issa, Filippo Paggetti and Ahmed El Damaty; methodology, Mohammed Issa, Filippo Paggetti and Ahmed El Damaty; software, Mohammed Issa and Filippo Paggetti; validation, Mohammed Issa, Filippo Paggetti Ahmed El Damaty and Andreas W. Unterberg; formal analysis, Mohammed Issa and Filippo Paggetti; investigation, Andreas W Unterberg; resources, Andreas Unterberg; data curation, Mohammed Issa, Filippo Paggetti, August von Hardenberg, Christoph Miethke and Ahmed El Damaty; writing—original draft preparation, Mohammed Issa, Filippo Paggetti and Ahmed El Damaty; writing—review and editing, Andreas W. Unterberg, Ahmed El Damaty; visualization, Ahmed El Damaty; supervision, Andreas W. Unterberg, Ahmed El Damaty; project administration, Ahmed El Damaty. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective, single-center study was approved by the institutional ethical committee (S-084/2022), and was conducted in concordance with the Declaration of Helsinki and its amendments.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent for publication
Obtained from coauthors after the agreement of all included patients.
Conflict of interest
August von Hardenberg and Christoph Miethke work at Miethke GmbH & Co. KG and supervised the valve analysis. The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous presentation: None.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Issa, M., Paggetti, F., von Hardenberg, A. et al. Programmable (proSA®) vs. fixed (SHUNTASSISTANT®) gravitational valves in pediatric patients with hydrocephalus: a 16-year retrospective single-center comparative study with biomechanical analysis. Acta Neurochir 165, 4031–4044 (2023). https://doi.org/10.1007/s00701-023-05751-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05751-y