Abstract
A new ratiometric fluorescent probe for efficient determination of ALP was developed. The probe was constructed by combining Ce3+-crosslinked copper nanoclusters (Ce3+-CuNCs) which exhibit the aggregation-induced emission (AIE) feature with carbon dots (CDs). The introduction of phosphate (Pi) induced the generation of CePO4 precipitation, resulting in significant decrease of fluorescence emission of CuNCs at 634 nm. At the same time, the fluorescence of CDs at 455 nm was obviously enhanced, thus generating ratiometric fluorescence response. Based on the fact that the hydrolysis of pyrophosphate (PPi) by ALP can produce Pi, the CD/Ce3+-CuNCs ratiometric probe was successfully used to determine ALP. A good linear relationship between the ratiometric value of F455/F634 and ALP concentrations ranging from 0.2 to 80 U·L− 1 was obtained, with a low detection limit of 0.1 U·L− 1. The ratiometric responses of the probe resulted in the visible fluorescence color change from orange red to blue with the increase of ALP concentration. The smartphone-based RGB recognition of the fluorescent sample images was used for ALP quantitative determination.
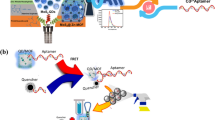
Graphical abstract

A novel ratiometric fluorescent system based on Ce3+-CuNCs with AIE feature and CDs were constructed for efficient detection of ALP.







Similar content being viewed by others
References
Ren XL, Yang LQ, Tang FQ, Yan CM, Ren J (2010) Enzyme biosensor based on NAD-sensitive quantum dots. Biosens Bioelectron 26(1):271–274. https://doi.org/10.1016/j.bios.2010.05.014
Sun T, Gao Y, Du Y, Zhou L, Chen X (2021) Recent advances in developing lanthanide metal-organic frameworks for ratiometric fluorescent sensing. Front Chem 8:624592. https://doi.org/10.3389/fchem.2020.624592
Colombatto P, Randone A, Civitico G, Monti Gorin J, Dolci L, Medaina N, Oliveri F, Verme G, Marchiaro G, Pagni R, Karayiannis P, Thomas HC, Hess G, Bonino F, Brunetto MR (1996) Hepatitis G virus RNA in the serum of patients with elevated gamma glutamyl transpeptidase and alkaline phosphatase: a specific Liver Disease. J Viral Hepatitis 3(6):301–306. https://doi.org/10.1111/j.1365-2893.1996.tb00102.x
Choi Y, Ho NH, Tung CH (2007) Sensing phosphatase activity by using gold nanoparticles. Angew Chem Int Edit 46(5):707–709. https://doi.org/10.1002/anie.200603735
Wei H, Chen C, Han B, Wang E (2008) Enzyme colorimetric assay using unmodified silver nanoparticles. Anal Chem 80(18):7051–7055. https://doi.org/10.1021/ac801144t
Liu Y, Schanze KS (2008) Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal Chem 80(22):8605–8612. https://doi.org/10.1021/ac801508y
Kazakevičienė B, Valinčius G, Kažemėkaitė M, Razumas V (2008) Self-assembled redox system for bioelectrocatalytic assay of L-ascorbylphosphate and alkaline phosphatase activity. Eletroanalysis 20(20):2235–2240. https://doi.org/10.1002/elan.200804316
Murata T, Yasukawa T, Shiku H, Matsue D (2009) Electrochemical single-cell gene-expression assay combining dielectrophoretic manipulation with secreted alkaline phosphatase reporter system. Biosens Bioelectron 25(4):913–919. https://doi.org/10.1016/j.bios.2009.09.001
Miao P, Ning L, Li X, Shu Y, Li G (2011) An electrochemical alkaline phosphatase biosensor fabricated with two DNA probes coupled with λ exonuclease. Biosens Bioelectron 27(1):178–182. https://doi.org/10.1016/j.bios.2011.06.047
Ino K, Kanno Y, Arai T, Inoue KY, Takahashi Y, Shiku H, Matsue T (2012) Novel electrochemical methodology for activity estimation of alkaline phosphatase based on solubility difference. Anal Chem 84(18):7593–7598. https://doi.org/10.1021/ac301429n
Huang L, Cao X, Gao T, Feng B, Huang X, Song R, Du T, Wen S, Feng X, Zeng W (2021) A novel aggregation-induced dual emission probe for in situ light-up detection of endogenous alkaline phosphatase. Talanta 225:121950. https://doi.org/10.1016/j.talanta.2020.121950
Chen Q, Bian N, Cao C, Qiu XL, Qi AD, Han BH Glucosamine hydrochloride functionalized tetraphenylethylene: a novel fluorescent probe for alkaline phosphatase based on the aggregation-induced emission. Chem Commun 46(23): 4067–4069. https://doi.org/10.1039/C002894K
Freeman R, Finder T, Gill R, Willner I (2010) Probing protein kinase (CK2) and Alkaline phosphatase with CdSe/ZnS Quantum dots. Nano Lett 10(6):2192–2196. https://doi.org/10.1021/nl101052f
Zhang L, Zhao J, Duan M, Zhang H, Jiang J, Ruqin Yu R (2013) Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal Chem 85(8):3797–3801. https://doi.org/10.1021/ac4001942
Nie F, Luo K, Zheng X, Zheng J, Song Z (2015) Novel preparation and electrochemiluminescence application of luminol functional-Au nanoclusters for ALP determination. Sens Actuat B-Chem 218:152–159. https://doi.org/10.1016/j.snb.2015.04.112
Sun D, Xu W, Liang C, Shi W, Xu S (2020) Smart surface-enhanced resonance raman scattering nanoprobe for monitoring cellular alkaline phosphatase activity during osteogenic differentiation. ACS Sens 5(6):1758–1767. https://doi.org/10.1021/acssensors.0c00428
Li D, Jiang Y, Chen S, Zhang Y, Wang W, Sun Y, Ma P, Song D, Wang X (2020) A simple and sensitive assay of alkaline phosphatase activity in serum by fluorescent silicon nanoparticles based on inner filter effect. Sens Actuat B-Chem 307:127589. https://doi.org/10.1016/j.snb.2019.127589
Li D, Qin J, Xu Q, Yan G (2018) A room-temperature phosphorescence sensor for the detection of alkaline phosphatase activity based on Mn-doped ZnS quantum dots. 274:78–84. https://doi.org/10.1016/j.snb.2018.07.108
Ma JL, Yin BC, Wu X, Ye BC (2016) Copper-mediated DNA-scaffolded silver nanocluster on-off switch for detection of pyrophosphate and alkaline phosphatase. Anal Chem 88(18):9219–9225. https://doi.org/10.1021/acs.analchem.6b02465
Qu F, Pei H, Kong R, Zhu S, Xia L (2017) Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta 165:136–142. https://doi.org/10.1016/j.talanta.2016.11.051
Zhan Y, Yang S, Chen L, Zeng Y, Li L, Lin Z, Guo L, Xu W (2021) Ultrahigh efficient FRET ratiometric fluorescence biosensor for visual detection of alkaline phosphatase activity and its inhibitor. ACS Sustainable Chem Eng 9:12922–12929. https://doi.org/10.1021/acssuschemeng.1c03830
Zhan Y, Yang J, Guo L, Luo F, Qiu B, Hong G, Lin Z (2019) Targets regulated formation of boron nitride quantum dots-gold nanoparticles nanocomposites for ultrasensitive detection of acetylcholinesterase activity and its inhibitors. Sens Actuat B-Chem 279:61–68. https://doi.org/10.1016/j.snb.2018.09.097
Chen BB, Liu ML, Gao YT, Chang S, Qian RC, Li DW (2023) Design and applications of carbon dots-based ratiometric fluorescent probes: a review. Nano Res 16:1064–1083. https://doi.org/10.1007/s12274-022-4840-2
Gui R, Jin H, Bu X, Fu Y, Wang Z, Liu Q (2019) Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coord Chem Rev 383:82–103. https://doi.org/10.1016/j.ccr.2019.01.004
An Y, Ren Y, Bick M, Dudek A, Waworuntu EHW, Tang J, Chen J, Chang B (2020) Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens Bioelectron 154:112078. https://doi.org/10.1016/j.bios.2020.112078
Liu X, Astruc D (2018) Atomically precise copper nanoclusters and their applications. Coord Chem Rev 359:112–126. https://doi.org/10.1016/j.ccr.2018.01.001
Jayasree M, Aparna RS, Anjana RR, Anjali Devi JS, John N, Abha K, Manikandan A, George S (2018) Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; a mechanistic perception. Anal Chim Acta 1031:152–160. https://doi.org/10.1016/j.aca.2018.05.026
Ouyang X, Wang M, Guo L, Cui C, Liu T, Ren Y, Zhao Y, Ge Z, Guo X, Xie G, Li J, Fan C, Wang L (2020) DNA nanoribbon-templated self-assembly of ultrasmall fluorescent copper nanoclusters with enhanced luminescence. Angew Chem Int Edit 59(29):11836–11844. https://doi.org/10.1002/anie.202003905
Maity S, Bain D, Patra A (2019) Engineering atomically precise copper nanoclusters with aggregation induced emission. J Phys Chem C 123(4):2506–2515. https://doi.org/10.1021/acs.jpcc.8b09467
Sabarinathan D, Sharma AS, Agyekum AA, Murugavelu M, Sabapathy PC, Ali S, Hassan H, Li H, Chen Q (2022) Thunnus albacares protein-mediated synthesis of water-soluble copper nanoclusters as sensitive fluorescent probe for Ferric ion detection. J Mol Struct 1254:132333. https://doi.org/10.1016/j.molstruc.2022.132333
Li D, Wang G, Cheng L, Wang C, Mei X (2018) Engineering the self-assembly induced emission of copper nanoclusters as 3D nanomaterials with mesoporous sphere structures by the crosslinking of Ce3+. ACS Omega 3(11):14755–14765. https://doi.org/10.1021/acsomega.8b02204
Zhang Q, Mei H, Zhou WT, Wang XD (2021) Cerium ion(III)-triggered aggregation-induced emission of copper nanoclusters for trace-level p-nitrophenol detection in water. Microchem J 162:105842. https://doi.org/10.1016/j.microc.2020.105842
Kong RM, Fu T, Sun N, Qu F, Zhang SF, Zhang XB (2013) Pyrophosphate-regulated Zn2+ -dependent DNAzyme activity: an amplified fluorescence sensing strategy for alkaline phosphatase. Biosens Bioelectron 50:351–355. https://doi.org/10.1016/j.bios.2013.06.064
Chen C, Zhao J, Lu Y, Sun J, Yang X (2018) Fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase. Anal Chem 90(5):3505–3511. https://doi.org/10.1021/acs.analchem.7b05325
Yan F, Bai Z, Liu F, Zu F, Zhang R, Xu J, Chen L (2018) Ratiometric fluorescence probes based on carbon dots. Curr Org Chem 22(1):57–66. https://doi.org/10.2174/1385272821666171005152058
Ma C, Li P, Xia L, Qu F, Kong RM, Song Z (2021) A novel ratiometric fluorescence nanoprobe for sensitive determination of uric acid based on CD@ZIF-CuNC nanocomposites. Microchim Acta 188(8):259. https://doi.org/10.1007/s00604-021-04914-x
Su X, Liu J (2017) pH-Guided self-assembly of copper nanoclusters with aggregation-induced emission. ACS Appl Mater Interfaces 9(4):3902–3910. https://doi.org/10.1021/acsami.6b13914
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020KB020), the National Natural Science Foundation of China (22074080), the Graduate Education Innovation Program of Qufu Normal University (CXJ1903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, RM., Li, P., Ge, X. et al. Ratiometric fluorescence determination of alkaline phosphatase activity based on carbon dots and Ce3+-crosslinked copper nanoclusters. Microchim Acta 190, 487 (2023). https://doi.org/10.1007/s00604-023-06048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06048-8