Abstract
The photoelectrochemical immunoassay of glycogen phosphorylase BB (GPBB) was studied. A methyl orange/TiO2 nanorod heterojunction was constructed on a fluorine-doped tin oxide electrode by hydrothermal synthesis, calcination, and chemical adsorption. A sandwich immune structure consisting of GPBB as the first antibody, GPBB, and a CdS@mesoporous silica-ascorbic acid (AA)–GPBB as secondary antibody composite was constructed on each of the selected well surfaces of a 96-well microplate. By adding mercaptoethylamine to structurally destroy the secondary antibody composite and release the electron donor AA, the amplification of photocurrent, and thus the “off–on” photoelectrochemical biosensing of GPBB were realized. The use of the 96-well microplate provides good reproducibility of the assembled immune structures and eliminates the possible effect of the photogenerated hole–induced protein oxidation on the photocurrent. The relevant electrodes and materials were characterized by electrochemistry, UV–vis diffuse reflectance spectra, Fourier transform infrared spectroscopy, X-ray diffractometer, scanning electron microscopy/energy dispersive spectroscopy, transmission electron microscopy and BET method. Under the optimal conditions, the photocurrent was linear with the logarithm of GPBB concentration from 0.005 to 200 ng mL−1 and with a limit of detection of 1.7 pg mL−1 (S/N = 3). Satisfactory results were obtained in the analysis of real serum samples.
Graphical abstract
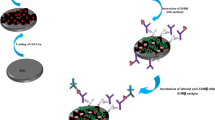
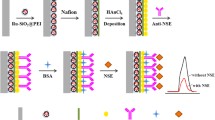
A sandwich immune structure consisting of GPBB first antibody, GPBB, and a CdS@mesoporous silica-ascorbic acid (AA)–GPBB secondary antibody composite was constructed on each of the selected well surfaces of a 96-well microplate. By adding mercaptoethylamine to structurally destroy the secondary antibody composite and release the electron donor AA, the amplification of photocurrent, and thus the “off–on” photoelectrochemical biosensing of GPBB were realized.







Similar content being viewed by others
References
Pu QL, Yang XH, Guo YC, Dai T, Yang TY, Ou XY et al (2019) Simultaneous colorimetric determination of acute myocardial infarction biomarkers by integrating self-assembled 3D gold nanovesicles into a multiple immunosorbent assay. Microchim Acta 186:138. https://doi.org/10.1007/s00604-019-3242-y
Aldous SJ (2013) Cardiac biomarkers in acute myocardial infarction. Int J Cardiol 164:282–294. https://doi.org/10.1016/j.ijcard.2012.01.081
Aydin S, Ugur K, Aydin S, Sahin I, Yardim M (2019) Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag 15:1–10. https://doi.org/10.2147/vhrm.S166157
Pyati AK, Devaranavadagi BB, Sajjannar SL, Nikam SV, Shannawaz M, Sudharani (2015) Heart-type fatty acid binding protein: a better cardiac biomarker than CK-MB and myoglobin in the early diagnosis of acute myocardial infarction. Journal of clinical and diagnostic research: JCDR 9:BC08-11. https://doi.org/10.7860/jcdr/2015/15132.6684
Nigam PK (2007) Biochemical markers of myocardial injury. Indian J Clin Biochem: IJCB 22:10–17. https://doi.org/10.1007/bf02912874
Lippi G, Mattiuzzi C, Comelli I, Cervellin G (2013) Glycogen phosphorylase isoenzyme BB in the diagnosis of acute myocardial infarction: a meta-analysis. Biochemia Medica 23:78–82. https://doi.org/10.11613/bm.2013.010
Apple FS, Wu AHB, Mair J, Ravkilde J, Panteghini M, Tate J et al (2005) Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem 51:810–824. https://doi.org/10.1373/clinchem.2004.046292
Krause EG, Rabitzsch G, Noll F, Mair J, Puschendorf B (1996) Glycogen phosphorylase isoenzyme BB in diagnosis of myocardial ischaemic injury and infarction. Mol Cell Biochem 160–161:289–295. https://doi.org/10.1007/BF00240061
Lim WY, Thevarajah TM, Goh BT, Khor SM (2019) Paper microfluidic device for early diagnosis and prognosis of acute myocardial infarction via quantitative multiplex cardiac biomarker detection. Biosens Bioelectron 128:176–185. https://doi.org/10.1016/j.bios.2018.12.049
Singh N, Rathore V, Mahat RK, Rastogi P (2018) Glycogen phosphorylase BB: a more sensitive and specific marker than other cardiac markers for early diagnosis of acute myocardial infarction. Indian J Clin Biochem 33:356–360. https://doi.org/10.1007/s12291-017-0685-y
Park K-Y, Ay I, Avery R, Caceres JA, Siket MS, Pontes-Neto OM et al (2018) New biomarker for acute ischaemic stroke: plasma glycogen phosphorylase isoenzyme BB. J Neurol Neurosurg Psychiatry 89:404–409. https://doi.org/10.1136/jnnp-2017-316084
Tabrizi MA, Ferre-Borrull J, Kapruwan P, Marsal LF (2019) A photoelectrochemical sandwich immunoassay for protein S100, a biomarker for Alzheimer’s disease, using an ITO electrode modified with a reduced graphene oxide-gold conjugate and CdS-labeled secondary antibody. Microchim Acta 186:117. https://doi.org/10.1007/s00604-018-3159-x
Zhao WW, Xu JJ, Chen HY (2015) Photoelectrochemical bioanalysis: the state of the art. Chem Soc Rev 44:729–741. https://doi.org/10.1039/c4cs00228h
Li N, Fu C, Wang F, Sun Y, Zhang L, Ge S et al (2020) Photoelectrochemical detection of let-7a based on toehold-mediated strand displacement reaction and Bi2S3 nanoflower for signal amplification. Sensors and Actuators B-Chemical 323:128665. https://doi.org/10.1016/j.snb.2020.128655
Wang GL, Yuan F, Gu TT, Dong YM, Wang Q, Zhao WW (2018) Enzyme-initiated quinone-chitosan conjugation chemistry: toward a general in situ strategy for high-throughput photoelectrochemical enzymatic bioanalysis. Anal Chem 90:1492–1497. https://doi.org/10.1021/acs.analchem.7b04625
Wang H, Xiao J, Li C, Li X, Deng K (2020) A photoelectrochemical immunosensor for prostate specific antigen detection based on graphdiyne oxide conjugated with horseradish peroxidase. Electroanalysis 33:652–662. https://doi.org/10.1002/elan.202060296
Yang WK, Wang XH, Hao WJ, Wu Q, Peng J, Tu JC et al (2020) 3D hollow-out TiO2 nanowire cluster/GOx as an ultrasensitive photoelectrochemical glucose biosensor. J Mat Chem B 8:2363–2370. https://doi.org/10.1039/d0tb00082e
Xu R, Liu L, Liu XJ, Li YY, Feng RQ, Wang H et al (2020) Novel electron donor encapsulation assay based on the split-type photoelectrochemical interface. ACS Appl Mater Interfaces 12:7366–7371. https://doi.org/10.1021/acsami.9b21804
Wang YH, Shi HH, Cui K, Zhang LN, Ge SG, Yan M et al (2018) Hierarchical hematite/TiO2 nanorod arrays coupled with responsive mesoporous silica nanomaterial for highly sensitive photoelectrochemical sensing. Biosens Bioelectron 117:515–521. https://doi.org/10.1016/j.bios.2018.06.030
Zhou Q, Lin YX, Shu J, Zhang KY, Yu ZZ, Tang DP (2017) Reduced graphene oxide-functionalized FeOOH for signal-on photoelectrochemical sensing of prostate-specific antigen with bioresponsive controlled release system. Biosens Bioelectron 98:15–21. https://doi.org/10.1016/j.bios.2017.06.033
Hafez H, Lan Z, Li Q, Wu J (2010) High efficiency dye-sensitized solar cell based on novel TiO2 nanorod/nanoparticle bilayer electrode. Nanotechnol Sci Appl 3:45–51. https://doi.org/10.2147/nsa.S11350
Guo WX, Xu C, Wang X, Wang SH, Pan CF, Lin CJ et al (2012) Rectangular bunched rutile TiO2 nanorod arrays grown on carbon fiber for dye-sensitized solar Cells. J Am Chem Soc 134:4437–4441. https://doi.org/10.1021/ja2120585
Asiam FK, Hao NH, Kaliamurthy AK, Kang HC, Yoo K, Lee JJ (2021) Preliminary investigation on vacancy filling by small molecules on the performance of dye-sensitized solar cells: the case of a type-II absorber. Front Chem 9:701781. https://doi.org/10.3389/fchem.2021.701781
Zhang KY, Lv SZ, Zhou Q, Tang DP (2020) CoOOH nanosheets-coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sens Actuators B-Chem 307:127631. https://doi.org/10.1016/j.snb.2019.127631
Lai C-Y, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S et al (2003) A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc 125:4451–4459. https://doi.org/10.1021/ja028650l
Zeng RJ, Luo ZB, Su LS, Zhang LJ, Tang DP, Niessner R et al (2019) Palindromic molecular beacon based Z-scheme BiOCI-Au-CdS photoelectrochemical biodetection. Anal Chem 91:2447–2454. https://doi.org/10.1021/acs.analchem.8b05265
Wang Y, Bian F, Qin XF, Wang QQ (2018) Visible light photoelectrochemical aptasensor for chloramphenicol by using a TiO2 nanorod array sensitized with Eu(III)-doped CdS quantum dots. Microchim Acta 185:161. https://doi.org/10.1007/s00604-018-2711-z
Liu Q, Lu H, Shi ZW, Wu FL, Guo J, Deng KM et al (2014) 2D ZnIn2S4 nanosheet/lD TiO2 nanorod heterostructure arrays for improved photoelectrochemical water splitting. ACS Appl Mater Interfaces 6:17200–17207. https://doi.org/10.1021/am505015j
Martindale BCM, Hutton GAM, Caputo CA, Reisner E (2015) Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J Am Chem Soc 137:6018–6025. https://doi.org/10.1021/jacs.5b01650
Suteewong T, Sai H, Bradbury M, Estroff LA, Gruner SM, Wiesner U (2012) Synthesis and formation mechanism of aminated mesoporous silica nanoparticles. Chem Mater 24:3895–3905. https://doi.org/10.1021/cm301857e
Li X, Zhou YL, Xu Y, Xu HJ, Wang MH, Yin HS et al (2016) A novel photoelectrochemical biosensor for protein kinase activity assay based on phosphorylated graphite-like carbon nitride. Anal Chim Acta 934:36–43. https://doi.org/10.1016/j.aca.2016.06.024
Wang BH, Qin Y, Tan WS, Tao YX, Kong Y (2017) Smartly designed 3D N-doped mesoporous graphene for high-performance supercapacitor electrodes. Electrochim Acta 241:1–9. https://doi.org/10.1016/j.electacta.2017.04.120
Sun C, Liu L, Guo C, Shen Y, Peng Y, Xie Q (2022) Photoelectrochemical biosensing of leukemia gene based on CdS/AuNPs/FeOOH Z-scheme heterojunction and a facile reflective device. Sens Actuators, B Chem 362:131795. https://doi.org/10.1016/j.snb.2022.131795
Rafigh SM, Heydarinasab A (2017) Mesoporous chitosan-SiO2 nanoparticles: synthesis, characterization, and CO2 adsorption capacity. ACS Sustain Chem Eng 5:10379–10386. https://doi.org/10.1021/acssuschemeng.7b02388
Lim WY, Goh CH, Thevarajah TM, Goh BT, Khor SM (2020) Using SERS-based microfluidic paper-based device (mu PAD) for calibration-free quantitative measurement of AMI cardiac biomarkers. Biosens Bioelectron 147:111792. https://doi.org/10.1016/j.bios.2019.111792
Funding
This work was supported by the National Natural Science Foundation of China (22074039, 21675050, 22002042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, C., Li, L., Liu, J. et al. Photoelectrochemical sandwich immunoassay of brain glycogen phosphorylase based on methyl orange–sensitized TiO2 nanorods. Microchim Acta 189, 265 (2022). https://doi.org/10.1007/s00604-022-05367-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05367-6