Abstract
A metal-organic framework nanocomposite was synthesized and applied to speciation analysis of Se(IV) and Se(VI). The sorbent is composed of MIL-101(Cr) and magnetite nanoparticles modified with dithiocarbamate. It is capably of selectively extracting Se(IV) at pH = 1.85, while Se(VI) remains in solution. The total amount of selenium can then be determined by reducing Se(VI) to Se(IV) and also extracting it. The extraction parameters were optimized by employing design-of-experiments methodology. Selenium was then quantified by electrothermal AAS. Figures of merit include (a) a 10 ng·L−1 limit of detection, (b) a linear response in the 30 ng·L−1 to 10 μg·L−1 concentration range, and (a) a relative standard deviation of <11.5% for Se(IV). The method was validated by analyzing certified reference materials (water and tomato leaves). It was also applied to the speciation analysis of Se(IV) and Se(VI) in (spiked) water samples and of total selenium in agricultural samples.

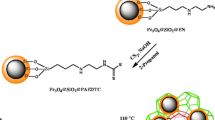
Schematic of the synthesis of a metal-organic framework nanocomposite for speciation analysis of Se(IV) and Se(VI). The sorbent is composed of MIL-101(Cr) and magnetite nanoparticles modified with dithiocarbamate. Selenium can be quantified by electrothermal AAS with a 10 ng L−1 detection limit.


Similar content being viewed by others
References
Shakerian F, Dadfarnia S, Haji Shabani AM, Shiralian Esfahani G (2013) Preconcentration and determination of lead(II) by microextraction based on suspended cadion covered zirconia nano-particles in a surfactant media. Microchim Acta 180:1225–1232
Zeng SL, Gan N, Weideman-Mera R, Cao YT, Li TH, Sang WG (2013) Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem Eng J 218:108–115
Asgharinezhad AA, Jalilian N, Ebrahimzadeh H, Panjali Z (2015) A simple and fast method based on new magnetic ion imprinted polymer nanoparticles for the selective extraction of Ni(II) ions in different food samples. RSC Adv 5:45510–45519
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA, Molaei K (2017) Extraction and determination of trace amounts of gold(III), palladium(II), platinum(II) and silver(I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim Acta 184:2191–2200
Tahmasebi E, Yamini Y (2014) Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver(I), gold(III), copper(II) and palladium(II). Microchim Acta 181:543–551
Cui Y, Liu S, Wei K, Liu Y, Hu Z (2015) Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim Acta 182:1337–1344
Mehdinia A, Shoormeij Z, Jabbari A (2017) Trace determination of lead(II) ions by using a magnetic nanocomposite of the type Fe3O4/TiO2/PPy as a sorbent, and FAAS for quantitation. Microchim Acta 184:1529–1537
Li QL, Wang LL, Wang X, Wang ML, Zhao RS (2016) Magnetic metal-organic nanotubes: an adsorbent for magnetic solid-phase extraction of polychlorinated biphenyls from environmental and biological samples. J Chromatogr A 1449:39–47
Yang Y, Ma X, Feng F, Dang X, Huang J, Chen H (2016) Magnetic solid-phase extraction of triclosan using core-shell Fe3O4@MIL-100 magnetic nanoparticles, and its determination by HPLC with UV detection. Microchim Acta 183:2467–2472
Kolaei M, Dashtian K, Rafiee Z, Ghaedi M (2016) Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT- Fe3O4-NPs: central composite design optimization. Ultrason Sonochem 33:240–248
Liu J, Zhao Z, Shao P, Cui F (2015) Activation of peroxy monosulfate with magnetic Fe3O4-MnO2 core-shell nanocomposites for 4-chlorophenol degradation. Chem Eng J 262:854–861
Rocío-Bautista P, Pacheco-Fernández I, Pasán J, Pino V (2016) Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?-a review. Anal Chim Acta 939:26–41
Maya F, Cabello CP, Frizzarin RM, Estela JM, Palomino GT, Cerdà V (2017) Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends Anal Chemistry 90:142–152
Saikia M, Bhuyan D, Saikia L (2015) Facile synthesis of Fe3O4 nanoparticles on metal organic framework MIL-101 (Cr): characterization and catalytic activity. New J Chem 39:64–67
Zhang Q, Yang G (2014) Selenium speciation in bay scallops by high performance liquid chromatography separation and inductively coupled plasma mass spectrometry detection after complete enzymatic extraction. J Chromatogr A 1325:83–91
Pan F, Tyson JF, Uden PC (2007) Simultaneous speciation of arsenic and selenium in human urine by high-performance liquid chromatography inductively coupled plasma mass spectrometry. J Anal Atom Spect 22:931–937
Grijalba AC, Martinis EM, Wuilloud RG (2017) Inorganic selenium speciation analysis in allium and brassica vegetables by ionic liquid assisted liquid-liquid microextraction with multivariate optimization. Food Chem 219:102–108
Wang RY, Hsu YL, Chang LF, Jiang SJ (2007) Speciation analysis of arsenic and selenium compounds in environmental and biological samples by ion chromatography-inductively coupled plasma dynamic reaction cell mass spectrometer. Anal Chim Acta 590:239–244
Combs GF, Combs SB (1986) The role of selenium in nutrition. Academic Press Inc, New York
Pinho J, Canario J, Cesario R, Vale CA (2005) Rapid acid digestion method with ICP-MS detection for the determination of selenium in dry sediments. Anal Chim Acta 551:207–212
Maplesden DC (1965) Selenium. Geobotany, biochemistry, toxicity and nutrition. Can Vet J 6:37
Stoecker BJ, Hopkins LL (1984) Vanadium. In: Frieden E (ed) Biochemistry of the Essential Ultratrace Elements. Biochemistry of the Elements, vol 3. Springer, Boston
Jagatap SP, Kolekar SS, Han SH, Anuse MA (2013) Liquid-liquid extraction of selenium(IV) and tellurium(IV) by N-n-octylcyclohexamine followed by their spectrophotometric determination. Res J Chem Sci 3:72–81
La Fuente JM, Sánchez ML, Marchante-Gayón JM, Uria JE, Sanz-Medel A (1996) On-line focused microwave digestion-hydride generation of inorganic and organic selenium: Total determination and inorganic selenium speciation by atomic absorption spectrometry. Spectrochim Acta Part B: Atom Spect 51:1849–1857
Maseko T, Callahan DL, Dunshea FR, Doronila A, Kolev SD, Ng K (2013) Chemical characterization and speciation of organic selenium in cultivated selenium-enriched Agaricusbisporus. Food Chem 141:3681–3687
Zhang Y, Duan J, He M, Chen B, Hu B (2013) Dispersive liquid-liquid microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for the speciation of inorganic selenium in environmental water samples. Talanta 115:730–736
Wang X, Chen P, Cao L, Xu G, Yang S, Fang Y, Wang G, Hong X (2017) Selenium speciation in Rice samples by magnetic ionic liquid-based up-and-down-shaker-assisted dispersive liquid-liquid microextraction coupled to graphite furnace atomic absorption spectrometry. Food Anal Methods 10:1653–1660
Lavi N, Alfassi ZB (1995) Determination of trace amounts of copper and selenium in biological samples by chemical separation prior to neutron activation analysis. J Radioanal Nucl Chem 201:13–23
Tuzen M, Saygi KO, Soylak M (2007) Separation and speciation of selenium in food and water samples by the combination of magnesium hydroxide coprecipitation-graphite furnace atomic absorption spectrometric determination. Talanta 71:424–429
Singh V, Agrawal HM (2012) Qualitative soil mineral analysis by EDXRF, XRD and AAS probes. Radiat Phys Chem 81:1796–1803
Sehar S, Naz I, Ali N, Ahmed S (2013) Analysis of elemental concentration using ICP-AES and pathogen indicator in drinking water of Qasim Abad, district Rawalpindi, Pakistan. Environ Monit Assess 185:1129–1135
Saygi KO, Melek E, Tuzen M, Soylak M (2007) Speciation of selenium(IV) and selenium(VI) in environmental samples by the combination of graphite furnace atomic absorption spectrometric determination and solid phase extraction on Diaion HP-2MG. Talanta 71:1375–1381
Zhang Y, Chen B, Wu S, He M, Hu B (2016) Graphene oxide-TiO2 composite solid phase extraction combined with graphite furnace atomic absorption spectrometry for the speciation of inorganic selenium in water samples. Talanta 154:474–480
Wang Y, Xie J, Wu Y, Hu X, Yang C, Xu Q (2013) Determination of trace amounts of se(IV) by hydride generation atomic fluorescence spectrometry after solid-phase extraction using magnetic multi-walled carbon nanotubes. Talanta 112:123–128
Yang W, Gao Y, Wu L, Hou X, Zheng C, Zhu X (2014) Preconcentration and in-situ photoreduction of trace selenium using TiO2 nanoparticles, followed by its determination by slurry photochemical vapor generation atomic fluorescence spectrometry. Microchim Acta 181:197–204
Abdolmohammad-Zadeh H, Jouyban A, Amini R, Sadeghi G (2013) Nickel-aluminum layered double hydroxide as a nano-sorbent for the solid phase extraction of selenium, and its determination by continuous flow HG-AAS. Microchim Acta 180:619–626
Wang Y, Luo X, Tang J, Hu X (2011) Determination of se (IV) using solidified floating organic drop microextraction coupled to ultrasound-assisted back-extraction and hydride generation atomic fluorescence spectrometry. Microchim Acta 173:267–273
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 488 kb)
Rights and permissions
About this article
Cite this article
Kalantari, H., Manoochehri, M. A nanocomposite consisting of MIL-101(Cr) and functionalized magnetite nanoparticles for extraction and determination of selenium(IV) and selenium(VI). Microchim Acta 185, 196 (2018). https://doi.org/10.1007/s00604-018-2731-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2731-8