Abstract
The authors describe a quartz crystal microbalance based aptasensor for the determination of Pb2+. In order to enhance its response, oligonucleotide-embellished gold nanoparticles (AuNPs) were used to amplify the frequency changes. The method is based on the use of specific aptamers immobilized on the surface of the quartz crystal microbalance (QCM) and the binding of Pb2+, which prevents the self-assembly of the AuNPs on the QCM. Trace concentrations of Pb2+ can be determined by monitoring the change in the response frequency of the QCM. The method has a 4 nmol∙L−1 detection limit and works in the 5 to 200 nmol∙L−1 Pb2+ concentration range. This aptasensor also shows adequate selectivity for Pb2+ over potentially interfering metal ions.

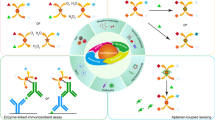
Schematic of the assay. The specific aptamer SAP does not readily combine with its partial complementary strand CSAP in the presence of Pb(II) ion. Thus, the mass change on the crystal is inversely proportional to the concentration of Pb(II).






Similar content being viewed by others
References
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Ghaedi M, Ahmadi F, Shokrollahi A (2007) Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J Hazard Mater 142:272–278
D'Ilio S, Petrucci F, D'Amato M, Di Gregorio M, Senofonte O, Violante N (2008) Method validation for determination of arsenic, cadmium, chromium and lead in milk by means of dynamic reaction cell inductively coupled plasma mass spectrometry. Anal Chim Acta 624:59–67
Zhai YX, Hao LH (2000) Determination of lead in food and feed samples using hydride generation-atomic fluorescence spectrometry. Chin J Anal Chem 28:176–179
Tan Y, Qiu JZ, Cui MY, Wei XF, Zhao MM, Qiu B, Chen GN (2016) An immobilization free DNAzyme based electrochemical biosensor for lead determination. Analyst 141:1121–1126
Bi ZS, Salaun P, van den Berg CMG (2013) Study of bare and mercury-coated vibrated carbon, gold and silver Microwire electrodes for the determination of lead and cadmium in seawater by anodic stripping voltammetry. Electroanal 25:357–366
He X, Chen L, Xie X, Su Z, Qin C, Liu Y, Ma M, Yao S, Deng L, Xie Q, Tian Y, Qin D, Luo Y (2012) Square wave anodic stripping voltammetric determination of lead(II) using a glassy carbon electrode modified with a lead ionophore and multiwalled carbon nanotubes. Microchim Acta 176:81–89
Yong W, Fan Y, Xiurong Y (2010) Colorimetric biosensing of mercury(II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens Bioelectron 25:1994–1998
Wu YG, Zhan SS, Xing HB, He L, Xu LR, Zhou P (2012) Nanoparticles assembled by aptamers and crystal violet for arsenic(III) detection in aqueous solution based on a resonance Rayleigh scattering spectral assay. Nanoscale 4:6841–6849
Li F, Yang L, Chen M, Qian Y, Tang B (2013) A novel and versatile sensing platform based on HRP-mimicking DNAzyme-catalyzed template-guided deposition of polyaniline. Biosens Bioelectron 41:903–906
Xie YF, Jin YL, Huang YY, Liu GQ, Zhao R (2014) Highly selective piezoelectric sensor for lead(II) based on the lead-catalyzed release of gold nanoparticles from a self-assembled nanosurface. Microchim Acta 181:1521–1527
Wang J, Zhu ZQ, Ma HW (2013) Label-free real-time detection of DNA methylation based on quartz crystal microbalance measurement. Anal Chem 85:2096–2101
Wang DZ, Tang W, Wu XJ, Wang XY, Chen GJ, Chen Q, Li N, Liu F (2012) Highly selective detection of single-nucleotide polymorphisms using a quartz crystal microbalance biosensor based on the toehold-mediated strand displacement reaction. Anal Chem 84:7008–7014
Duner G, Anderson H, Pei ZC, Ingemarsson B, Aastrup T, Ramstrom O (2016) Signal enhancement in ligand-receptor interactions using dynamic polymers at quartz crystal microbalance sensors. Analyst 141:3993–3996
Xu LZ, Wang RH, Kelso LC, Ying YB, Li YB (2016) A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N1. Sensor Actuat B-Chem 234:98–108
Yuan Z, Tai HL, Ye ZB, Liu CH, Xie GZ, Du XS, Jiang YD (2016) Novel highly sensitive QCM humidity sensor with low hysteresis based on graphene oxide (GO)/poly(ethyleneimine) layered film. Sensor Actuat B-Chem 234:145–154
Zhou YY, Tang L, Zeng GM, Zhang C, Xie X, Liu YY, Wang JJ, Tang J, Zhang Y, Deng YC (2016) Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon-gold nanoparticles and DNAzyme catalytic beacons. Talanta 146:641–647
Zhou L, Lu P, Zhu MY, Li BL, Yang PH, Cai JY (2016) Silver nanocluster based sensitivity amplification of a quartz crystal microbalance gene sensor. Microchim Acta 183:881–887
Dong Z-M, Zhao G-C (2012) Quartz crystal microbalance aptasensor for sensitive detection of mercury(II) based on signal amplification with gold nanoparticles. Sensors 12:7080–7094
Van Noort D, Rani R, Mandenius CF (2001) Improving the sensitivity of a quartz crystal microbalance for biosensing by using porous gold. Microchim Acta 136:49–53
Cheng S, Zheng B, Wang M, Ge X, Zhao Q, Liu W, Lam MH-W (2014) The unfolding of G-quadruplexes and its adverse effect on DNA-gold nanoparticles-based sensing system. Biosens Bioelectron 53:479–485
Liu W, Zhu H, Zheng B, Cheng S, Fu Y, Li W, Lau TC, Liang HJ (2012) Kinetics and mechanism of G-quadruplex formation and conformational switch in a G-quadruplex of PS2.M induced by Pb2+. Nucleic Acids Res 40:4229–4236
Kwan ICM, Mo X, Wu G (2007) Probing hydrogen bonding and ion-carbonyl interactions by solid-state O-17 NMR spectroscopy: G-ribbon and G-quartet. J Am Chem Soc 129:2398–2407
Teh HB, Li H, Li SFY (2014) Highly sensitive and selective detection of Pb2+ ions using a novel and simple DNAzyme-based quartz crystal microbalance with dissipation biosensor. Analyst 139:5170–5175
Babacan S, Pivarnik P, Letcher S, Rand AG (2000) Evaluation of antibody immobilization methods for piezoelectric biosensor application. Biosens Bioelectron 15:615–621
Wang XF, Xiang LP, Wang YS, Xue JH, Zhu YF, Huang YQ, Chen SH, Tang X (2016) A “turn-on” fluorescence assay for lead(II) based on the suppression of the surface energy transfer between acridine orange and gold nanoparticles. Microchim Acta 183:1333–1339
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 61501295 and No. 31671934), Shanghai Committee of Science and Technology (Grant No.15YF1408000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yuan, M., Song, Z., Fei, J. et al. Aptasensor for lead(II) based on the use of a quartz crystal microbalance modified with gold nanoparticles. Microchim Acta 184, 1397–1403 (2017). https://doi.org/10.1007/s00604-017-2135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2135-1