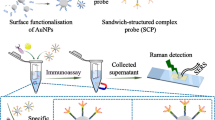
Abstract
A chemiluminescent immunoassay for the staphylococcal enterotoxin C1 (SEC1) based on the use of dye-encapsulated mesoporous silica nanoparticles (m-SiNPs) as a label is described. The dyes are retained in the m-SiNPs via strong hydrophobic interactions. The assay comprises the following steps: (a) Microplates coated with antibody against SEC1 are filled with sample upon which the SEC antigen will be bound to the surface; (b) following a washing step, secondary antibody linked to m-SiNPs (that were covalently labeled with rhodamine 6G and fluorescein) were added to form the sandwich complex; (c) after another washing step, bis(2,4,6-trichlorophenyl) oxalate, H2O2 and imidazole are added to generate chemiluminescence whose intensity is proportional to the number of m-SiNPs and thus to the number of antigen (SEC) molecules. It is found that the use of functionalized m-SiNPs strongly amplifies the signal. Enterotoxin SEC1 can be detected by this method in the 0.025 to 2 ng⋅mL‾1 concentration range, the detection limit is 19 pg⋅mL‾1 (at 3σ), and the relative standard deviation (for 11 parallel measurements at a 1 ng⋅mL‾1 level) is 4.6 %. The use of an automated chemiluminescence analyzer further improves detection.

A chemiluminescent immunoassay for the staphylococcal enterotoxin C1 (SEC1) is developed based on dye-encapsulated mesoporous silica nanoparticles (m-SiNPs) and bis(2,4,6-trichlorophenyl) oxalate (TCPO)-H2O2-dyes chemiluminescent system. The advantage of this method is low detection limit to 19 pg⋅mL‾1.




Similar content being viewed by others
References
Lee WM (1997) Hepatitis B virus infection. N Engl J Med 337:1733
Hedlund G, Dohlsten M, Petrsson C (1995) Superantigen induce cytokinc suppress growth of human coloncarcinomacels. Cancer Immucnol Immunother 37:89
Dohlsten M, Sundstedt A, Bjorklund M (1993) Superantigen-induced cytokines suppress growth of human colon-carcinoma cells. Cancer 54:482
White J, Herman A, Pullen AM (1989) The v beta specific superantigen staphylococcal enterotoxin B: stimulation of mature t cells and clonal deletion in neonatal mice. Cell 56:27
Bohach GA, Kreiswirth BN, Novick RP, Schlievert PM (1989) Analysis of toxic shock syndrome isolates producing staphylococcal enterotoxins B and C1 with use of southern hybridization and immunologic assays. Rev Infect Dis 11(Suppl 1):S75
Bohach GA, Fast DJ, Nelson RD, Schlievert PM (1990) Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol 17:251
Zorgani A, Essery SD, Madani OA, Bentley AJ, James VS, MacKenzie DA, Keeling JW, Rambaud C, Hilton J, Blackwell CC (1999) Detection of pyrogenic toxins of Staphylococcus aureus in sudden infant death syndrome. FEMS Immunol Med Microbiol 25:103
Mäntynen V, Niemelä S, Kaijalainen S, Pirhonen T, Lindström KV (1997) MPN-PCR-quantification method for staphylococcal enterotoxin c1 gene from fresh cheese. Int J Food Microbiol 36:135
Giletto A, Fyffe JG (1998) A novel ELISA format for the rapid and sensitive detection of staphylococcal enterotoxin A. Biosci Biotechnol Biochem 62:2217
Kientz CE, Hulst AG, Wils ER (1997) Determination of staphylococcal enterotoxin B by on-line (micro) liquid chromatography-electrospray mass spectrometry. J Chromatogr A 757(1–2):51
Rasooly A, Ito Y (1999) Toroidal coil countercurrent chromatography separation and analysis of staphylococcal enterotoxin A (SEA) in milk. J Liq Chromatogr Relat Technol 22:1285
Ligler FS, Taitt CR, Shriver-Lake LC, Sapsford KE, Shubin Y, Golden JP (2003) Array biosensor for detection of toxins. Anal Bioanal Chem 377(3):469
Rowe-Taitt CA, Golden JP, Feldstein MJ, Cras JJ, Hoffman KE, Ligler FS (2000) Array biosensor for detection of biohazards. Biosens Bioelectron 14:785
Dong S, Luo G, Feng J, Li Q, Gao H (2001) Immunoassay of staphylococcal enterotoxin C1 by FTIR spectroscopy and electrochemical gold electrode. Electroanalysis 13:30
Goldman ER, Balighian ED, Mattoussi H, Kuno MK, Mauro JM, Tran PT, Anderson GP (2002) Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc 124:6378
Lin HC, Tsai WC (2003) Piezoelectric crystal immunosensor for the detection of staphylococcal enterotoxin B. Biosens Bioelectron 18:1479
Luo LR, Zhang ZJ, Chen LJ, Ma LF (2006) Chemiluminescent imaging detection of staphylococcal enterotoxin C1 in milk and water samples. Food Chem 97:355
Hun X, Zhang ZJ (2007) A novel sensitive staphylococcal enterotoxin C1 fluoroimmunoassay based on functionalized fluorescent core-shell nanoparticle labels. Food Chem 105:1623
Huang Y, Zhang H, Chen X, Wang X, Duan N, Wu S, Xu B, Wang Z (2015) A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens Bioelectron 74:170
Hartmann M (2005) Ordered mesoporous materials for bioadsorption and biocatalysis. Chem Mater 17:4577
Yiu HHP, Wright PA (2005) Enzymes supported on ordered mesoporous solids: a special case of an inorganic–organic hybrid. J Mater Chem 15:3690
Lei C, Soares TA, Shin Y, Liu J, Ackerman EJ (2008) Enzyme specific activity in functionalized nanoporous supports. Nanotechnology 19:125102
Vinu A, Miyahara M, Ariga K (2006) Assemblies of biomaterials in mesoporous media. J Nanosci Nanotechnol 6:1510
Liu W, Ma C, Yang H, Zhang Y, Yan M, Ge S, Song X (2014) Electrochemiluminescence immunoassay using a paper electrode incorporating porous silver and modified with mesoporous silica nanoparticles functionalized with blue-luminescent carbon dots. Microchim Acta 181(11–12):1415
Roda A, Mirasoli M, Roda B, Bonvicini F, Colliva C, Reschiglian P (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178:7
Bamrungsap S, Apiwat C, Chantima W, Dharakul T, Wiriyachaiporn N (2014) Rapid and sensitive lateral flow immunoassay for influenza antigen using fluorescently-doped silica nanoparticles. Microchim Acta 181(1–2):223
Cho EB, Volkov DO, Sokolov I (2010) Ultrabright fluorescent mesoporous silica nanoparticles. Small 6:2314
Tao L, Zhang K, Sun YJ, Jin BQ, Zhang ZJ, Yang K (2012) Anti-epithelial cell adhesion molecule monoclonal antibody conjugated fluorescent nanoparticle biosensor for sensitive detection of colon cancer cells. Biosens Bioelectron 35:186
Tao L, Song CJ, Sun YJ, Li XH, Li YY, Jin BQ, Zhang ZJ, Yang K (2013) A fluorescent and chemiluminescent difunctional mesoporous silica nanoparticle as a label for the ultrasensitive detection of cancer cells. Anal Chim Acta 761:194
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant 81171977 and 31500614) and China Postdoctoral Science Foundation (2014 M562598).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liang Tao, Chunmei Zhang and Jinpeng Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 111 kb)
Rights and permissions
About this article
Cite this article
Tao, L., Zhang, C., Zhang, J. et al. Sensitive chemiluminescence immunoassay for staphylococcal enterotoxin C1 based on the use of dye-encapsulated mesoporous silica nanoparticles. Microchim Acta 183, 2163–2168 (2016). https://doi.org/10.1007/s00604-016-1849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1849-9