Abstract
Aims
Molecular defects of hepatic nuclear factor 1β (HNF1β) are associated with multiorgan disease (renal disease, pancreatic hypoplasia, and genital tract anomalies) in addition to diabetes. We examined the phenotypic features, insulin secretory response to glucose, and response to treatment in subjects with HNF1β-MODY (MODY 5).
Methods
Twelve subjects with HNF1β-MODY were phenotyped in detail. A 2-h oral glucose tolerance test was performed to establish insulin secretory response with glucose, insulin and C-peptide measurements taken at baseline and 30 min intervals. Clinical follow-up occurred bi-annually.
Results
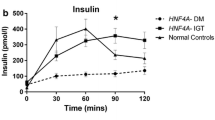
Ten of 12 subjects had diabetes with mean age of onset of 30.2 ± 15.5 years, fasting glucose of 9.7 ± 4.6 mmol/L and HbA1c of 60.9 ± 17.1 mmol/mol (7.7 ± 1.6%). Renal and/or pancreatic morphological abnormalities were found in 9 subjects. Mean fasting C-peptide (0.5 ± 0.4 nmol/L) and AUC C-peptide (1.5 ± 1.0 nmol/L/120 min) were reduced in our cohort with 4 subjects demonstrating marked insulin deficiency. OGIS was reduced at 290.2 ± 67.0 ml min−1 m−2. 6/10 subjects were on insulin therapy at initial diagnosis and 8/10 at last clinical follow-up. Mean insulin dose at last clinical follow-up was 0.45 ± 0.23units/kg/day. 5 subjects on insulin were trialled on sulphonylurea therapy, and none was successfully weaned off insulin.
Conclusions
Diagnosing HNF1β-MODY in a diabetes clinic is challenging due to its variable phenotype and variable age of onset. β-Cell dysfunction and insulin resistance contribute to diabetes in HNF1β-MODY. No subjects successfully transitioned to sulphonylurea. Early initiation of insulin therapy would be suitable to achieve glycaemic control. This emphasizes the importance of genetic testing for monogenic forms of diabetes to guide personalized treatment.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Fajans SS, Bell GI, Polonsky KS (2001) Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 345(13):971–980. https://doi.org/10.1056/NEJMra002168
Shepherd M, Shields B, Hammersley S, et al. (2016) United team systematic population screening, using biomarkers and genetic testing, identifies 25% of the UK pediatric diabetes population with monogenic diabetes. Diabetes Care. https://doi.org/10.2337/dc16-0645
Delvecchio M, Mozzillo E, Salzano G, et al. (2017) Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (ISPED). Monogenic Diabetes Accounts for 6.3% of Cases Referred to 15 Italian Pediatric Diabetes Centers During 2007 to 2012. J Clin Endocrinol Metab, https://doi.org/10.1210/jc.2016-2490.
Edghill EL, Stals K, Oram RA, et al (2013) HNF1B deletions in patients with young-onset diabetes but no known renal disease. Diabet Med 30(1):114–117. https://doi.org/10.1111/j.1464-5491.2012.03709.x
Kleinberger JW, Pollin TI (2015) Undiagnosed MODY: Time for Action. Curr DiabRep 15(12):110. https://doi.org/10.1007/s11892-015-0681-7
Bohn S, Thomas H, Turan G, et al. (2003) Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. Clin J Am Soc Nephrol 14(8):2033–2041. https://doi.org/10.1097/01.asn.0000078808.70309.c4
Heidet L, Decramer S, Pawtowski A, et al. (2010) Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol 5(6):1079–1090. https://doi.org/10.2215/CJN.06810909
Horikawa Y, Iwasaki N, Hara M, et al. (1997) Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet 17(4):384–385. https://doi.org/10.1038/ng1297-384
Haldorsen IS, Vesterhus M, Raeder H,et al. (2008) Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet Med 25(7):782–787. https://doi.org/10.1111/j.1464-5491.2008.02460.x
Lindner TH, Njolstad PR, Horikawa Y, et al. (1999) A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet 8(11):2001–2008. https://doi.org/10.1093/hmg/8.11.2001
Iwasaki N, Ogata M, Tomonaga O, et al. (1998) Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care 21(12):2144–2148. https://doi.org/10.2337/diacare.21.12.2144
Adalat S, Woolf AS, Johnstone KA, et al. (2009) HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. Clini J Am Soci Nephrol 20(5):1123–1131
Ferrè S, Bongers EM, Sonneveld R, et al. (2013) Early development of hyperparathyroidism due to loss of PTH transcriptional repression in patients with HNF1β mutations? J Clin Endocrinol Metab 98(10):4089–4096. https://doi.org/10.1210/jc.2012-3453
Loirat C, Bellanné-Chantelot C, Husson I, et al. (2010) Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrol Dial Transplant 25(10):3430–3433. https://doi.org/10.1093/ndt/gfq380
Bingham C, Bulman MP, Ellard S, et al. (2001) Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet 68(1):219–224. https://doi.org/10.1086/316945
Kolatsi-Joannou M, Bingham C, Ellard S, et al. (2001) Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. Clini J Am Soci Nephrol 12(10):2175–2180
Nishigori H, Yamada S, Kohama T, et al. (1998) Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1beta gene associated with diabetes and renal dysfunction. Diabetes 47(8):1354–1355. https://doi.org/10.2337/diab.47.8.1354
Dubois-Laforgue D, Cornu E, Saint-Martin C, et al. (2017) Monogenic Diabetes Study Group of the Société Francophone du Diabète Diabetes, Associated Clinical Spectrum, Long-term Prognosis, and Genotype/Phenotype Correlations in 201 Adult Patients With Hepatocyte Nuclear Factor 1B (HNF1B) Molecular Defects. Diabetes Care 40(11):1436–1443
Brackenridge A, Pearson ER, Shojaee-Moradie F, et al. (2006) Contrasting insulin sensitivity of endogenous glucose production rate in subjects with hepatocyte nuclear factor-1beta and -1alpha mutations. Diabetes 55(2):405–411. https://doi.org/10.2337/diabetes.55.02.06.db05-1019
Pearson ER, Badman MK, Lockwood CR, et al. (2004) Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1alpha and -1beta mutations. Diabetes Care 27(5):1102–1107. https://doi.org/10.2337/diacare.27.5.1102
Horikawa Y, Enya M, Fushimi N, et al. (2014) Screening of diabetes of youth for hepatocyte nuclear factor 1 mutations: clinical phenotype of HNF1β-related maturity-onset diabetes of the young and HNF1α-related maturity-onset diabetes of the young in Japanese. Diabet Med 31(6):721–727. https://doi.org/10.1111/dme.12416
Hattersley AT, Greeley SAW, Polak M, et al. (2018) ISPAD clinical practice consensus guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 19(Suppl 27):47–63. https://doi.org/10.1111/pedi.12772
Carrillo E, Lomas A, Pinés PJ, et al. (2017) Long-lasting response to oral therapy in a young male with monogenic diabetes as part of HNF1B-related disease. Endocrinol Diabetes Metab Case Rep 23(2017):17–0052. https://doi.org/10.1530/EDM-17-0052
Jones AG, Hattersley AT (2013) The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 30(7):803–817. https://doi.org/10.1111/dme.12159
Mari A, Pacini G, Murphy E, et al. (2001) A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. https://doi.org/10.2337/diacare.24.3.539
Shields BM, Hicks S, Shepherd MH, et al. (2010) Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 53(12):2504–2508
Isomaa B, Henricsson M, Lehto M, et al. (1998) Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia 41(4):467–473. https://doi.org/10.1007/s001250050931
Kyithar MP, Bacon S, Pannu KK, et al. (2011) Identification of HNF1A-MODY and HNF4A-MODY in Irish families: phenotypic characteristics and therapeutic implications. Diabetes Metab 37(6):512–519. https://doi.org/10.1016/j.diabet.2011.04.002
Bacon S, Kyithar MP, Rizvi SR, et al. (2016) Successful maintenance on sulphonylurea therapy and low diabetes complication rates in a HNF1A-MODY cohort. Diabet Med 33(7):976–984. https://doi.org/10.1111/dme.12992
Johansen A, Ek J, Mortensen HB, et al. (2005) Half of Clinically Defined Maturity-Onset Diabetes of the Young Patients in Denmark Do Not Have Mutations in HNF4A, GCK, and TCF1. J Clin Endocrinol Metab 90(8):4607–4614. https://doi.org/10.1210/jc.2005-0196
Ulinski T, Lescure S, Beaufils S, et al. (2006) Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. Clin J Am Soci Nephrol 17(2):497–503. https://doi.org/10.1681/ASN.2005101040
Maestro MA, Boj SF, Luco RF, et al. (2003) Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum Mol Genet 12(24):3307–3314. https://doi.org/10.1093/hmg/ddg355
De Vas MG, Kopp JL, Heliot C, et al. (2015) Hnf1b controls pancreas morphogenesis and the generation of Ngn3+ endocrine progenitors. Development 142(5):871–882. https://doi.org/10.1242/dev.110759
Wang L, Coffinier C, Thomas MK, et al. (2004) Selective deletion of the Hnf1beta (MODY5) gene in beta-cells leads to altered gene expression and defective insulin release. Endocrinology 145(8):3941–3949. https://doi.org/10.1210/en.2004-0281
Long Z, Cao M, Su S, et al. (2017) Inhibition of hepatocyte nuclear factor 1b induces hepatic steatosis through DPP4/NOX1-mediated regulation of superoxide. Free Radical Biol Med 113:71–83. https://doi.org/10.1016/j.freeradbiomed.2017.09.016
Hattersley AT, Patel KA (2017) Precision diabetes: learning from monogenic diabetes. Diabetologia 60(5):769–777. https://doi.org/10.1007/s00125-017-4226-2
Murphy R, Ellard S, Hattersley AT (2008) Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 4(4):200–213. https://doi.org/10.1038/ncpendmet0778
Pearson ER, Starkey BJ, Powell RJ, et al. (2003) Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 362(9392):1275–1281. https://doi.org/10.1016/S0140-6736(03)14571-0
Mateus JC, Rivera C, O’Meara M et al (2020) Maturity-onset diabetes of the young type 5 a MULTISYSTEMIC disease: a CASE report of a novel mutation in the HNF1B gene and literature review. Clin Diabetes Endocrinol 6:16. https://doi.org/10.1186/s40842-020-00103-6
Tao T, Yang Y, Hu Z (2020) A novel HNF1B mutation p.R177Q in autosomal dominant tubulointerstitial kidney disease and maturity-onset diabetes of the young type 5: A pedigree-based case report. Medicine (Baltimore) 99(31):21438
Roehlen N, Hilger H, Stock F, et al. (2018) 17q12 Deletion syndrome as a rare cause for diabetes mellitus type MODY5. J Clin Endocrinol Metab 103(10):3601–3610. https://doi.org/10.1210/jc.2018-00955
Warncke K, Kummer S, Raile K, G et al (2019) Frequency and characteristics of MODY 1 (HNF4A Mutation) and MODY 5 (HNF1B Mutation): Analysis From the DPV Database. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-01696
Lopes AM, Teixeira S (2021) New-onset diabetes after kidney transplantation revealing HNF1B-associated disease. Endocrinol Diabetes Metab Case Rep 27(2021):20–0165. https://doi.org/10.1530/EDM-20-0165
Acknowledgements
The authors wish to thank the subjects who participated in this study and the Consultant Endocrinologists who referred subjects to the Mater MODY clinic. We would like to acknowledge the laboratory staff in the Mater Misericordiae University hospital with a particular thanks to Ms.Rachel Cullen. We would also like to thank Dr.Kevin Colclough in the Exeter Molecular Genetics laboratory for genetic analysis and advice.
Funding
This study was sponsored by the HRB/MRCG Research Award RP-06/01. J.Kim was supported by an Irish Endocrine Society summer student research bursary, 2019.
Author information
Authors and Affiliations
Contributions
NN and MB were responsible for the study design. Material preparation, data collection and analysis were performed by NN, MBMZ, NS, JK and MB. The first draft of the manuscript was written by NN, MBMZ and MMB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Research Ethics Committee of the Mater University hospital (reference number 1/378/1021).
Consent to participate
Informed consent was obtained from all individual subjects included in the study.
Consent for publication
Informed consent was obtained from all individual subjects for publication of their data.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ng, N., Mijares Zamuner, M., Siddique, N. et al. Genotype–phenotype correlations and response to glucose lowering therapy in subjects with HNF1β associated diabetes. Acta Diabetol 59, 83–93 (2022). https://doi.org/10.1007/s00592-021-01794-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01794-8