Abstract
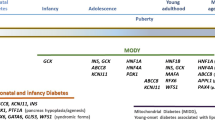
Maturity-onset diabetes of the young (MODY) is a monogenic form of diabetes that accounts for at least 1 % of all cases of diabetes mellitus. MODY classically presents as non-insulin-requiring diabetes in lean individuals typically younger than 25 with evidence of autosomal dominant inheritance, but these criteria do not capture all cases and can also overlap with other diabetes types. Genetic diagnosis of MODY is important for selecting the right treatment, yet ~95 % of MODY cases in the USA are misdiagnosed. MODY prevalence and characteristics have been well-studied in some populations, such as the UK and Norway, while other ethnicities, like African and Latino, need much more study. Emerging next-generation sequencing methods are making more widespread study and clinical diagnosis increasingly feasible; at the same time, they are detecting other mutations in the same genes of unknown clinical significance. This review will cover the current epidemiological studies of MODY and barriers and opportunities for moving toward a goal of access to an appropriate diagnosis for all affected individuals.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi:10.2337/dc15-S005.
Yamagata K, Furuta H, Oda N, et al. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384(6608):458–60.
Vionnet N, Stoffel M, Takeda J, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356(6371):721–2.
Yamagata K, Oda N, Kaisaki PJ, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384(6608):455–8.
Stoffers DA, Ferrer J, Clarke WL, et al. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17(2):138–9.
Horikawa Y, Iwasaki N, Hara M, et al. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17(4):384–5.
Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23(3):323–8.
Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102(13):4807–12.
Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38(1):54–62.
Plengvidhya N, Kooptiwut S, Songtawee N, et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007;92(7):2821–6.
Edghill EL, Flanagan SE, Patch AM, et al. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57(4):1034–42.
Borowiec M, Liew CW, Thompson R, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106(34):14460–5.
Bowman P, Flanagan SE, Edghill EL, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012;55(1):123–7.
Bonnefond A, Philippe J, Durand E, et al. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS One. 2012;7(6):e37423.
Hattersley A, Bruining J, Shield J, et al. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009;10 Suppl 12:33–42.
Shepherd M, Shields B, Ellard S, et al. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009;26(4):437–41.
Pearson ER, Liddell WG, Shepherd M, et al. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med. 2000;17(7):543–5.
Steele AM, Shields BM, Wensley KJ, et al. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014;311(3):279–86.
Ajjan RA, Owen KR. Glucokinase MODY and implications for treatment goals of common forms of diabetes. Curr Diab Rep. 2014;14(12):559,014-0559-0.
Shepherd M, Hattersley AT. ‘I don’t feel like a diabetic any more’: the impact of stopping insulin in patients with maturity onset diabetes of the young following genetic testing. Clin Med. 2004;4(2):144–7.
Bosma AR, Rigter T, Weinreich SS, Cornel MC, Henneman L. A genetic diagnosis of maturity-onset diabetes of the young (MODY): experiences of patients and family members. Diabet Med. 2015;32(10):1385–92. doi:10.1111/dme.12742.
Naylor RN, John PM, Winn AN, et al. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37(1):202–9. The authors use economic models that incorporate test cost, prevalence and natural history data to evaluate the cost-effectiveness of testing young adults with a diagnosis of type 2 diabetes for the three most common types of MODY (HNF-1A, HNF4A and GCK) and yield evidence that testing is cost-effective to cost-saving, depending upon prevalence.
Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–77.
Mak CM, Lee CY, Lam CW, et al. Personalized medicine switching from insulin to sulfonylurea in permanent neonatal diabetes mellitus dictated by a novel activating ABCC8 mutation. Diagn Mol Pathol. 2012;21(1):56–9.
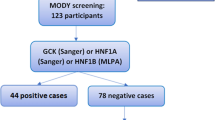
Shields BM, Hicks S, Shepherd MH, et al. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504–8.
Thanabalasingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35(6):1206–12. This exploration of the value of testing for MODY gene mutations among individuals with a diagnosis of type 1 or type 2 diabetes demonstrates that systematic testing would increase the number of MODY diagnoses, particularly in cases of an initial diagnosis of type 2 diabetes. The authors conclude with the recommendation that all patients diagnosed before age 30 with C-peptide at three years duration be considered for MODY testing.
Weinreich SS, Bosma A, Henneman L, et al. A decade of molecular genetic testing for MODY: a retrospective study of utilization in The Netherlands. Eur J Hum Genet. 2015;23(1):29–33.
Søvika O, Irgens HU, Molnes J, et al. Monogenic diabetes mellitus in Norway. Norw J Epidemiol. 2013;23(1):55–60.
Irgens HU, Molnes J, Johansson BB, et al. Prevalence of monogenic diabetes in the population-based Norwegian childhood diabetes registry. Diabetologia. 2013;56(7):1512–9.
Schober E, Rami B, Grabert M, et al. Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med. 2009;26(5):466–73.
Ellard S, Bellanne-Chantelot C, Hattersley AT, et al. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51(4):546–53.
Fendler W, Borowiec M, Baranowska-Jazwiecka A, et al. Prevalence of monogenic diabetes amongst Polish children after a nationwide genetic screening campaign. Diabetologia. 2012;55(10):2631–5.
Borowiec M, Fendler W, Antosik K, et al. Doubling the referral rate of monogenic diabetes through a nationwide information campaign—update on glucokinase gene mutations in a Polish cohort. Clin Genet. 2012;82(6):587–90.
Mozzillo E, Salzano G, Barbetti F, et al. Survey on etiological diagnosis of diabetes in 1244 Italian diabetic children and adolescents: impact of access to genetic testing. Diabetes Res Clin Pract. 2015;107(3):e15–8.
Johansen A, Ek J, Mortensen HB, et al. Half of clinically defined maturity-onset diabetes of the young patients in Denmark do not have mutations in HNF4A, GCK, and TCF1. J Clin Endocrinol Metab. 2005;90(8):4607–14.
Lorini R, Klersy C, d’Annunzio G, et al. Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care. 2009;32(10):1864–6.
Delvecchio M, Ludovico O, Menzaghi C, et al. Low prevalence of HNF1A mutations after molecular screening of multiple MODY genes in 58 Italian families recruited in the pediatric or adult diabetes clinic from a single Italian hospital. Diabetes Care. 2014;37(12):e258-60.
Massa O, Meschi F, Cuesta-Munoz A, et al. High prevalence of glucokinase mutations in Italian children with MODY. Influence on glucose tolerance, first-phase insulin response, insulin sensitivity and BMI. Diabetologia. 2001;44(7):898–905.
Capuano M, Garcia-Herrero CM, Tinto N, et al. Glucokinase (GCK) mutations and their characterization in MODY2 children of southern Italy. PLoS One. 2012;7(6):e38906.
Pruhova S, Ek J, Lebl J, et al. Genetic epidemiology of MODY in the Czech Republic: new mutations in the MODY genes HNF-4alpha, GCK and HNF-1alpha. Diabetologia. 2003;46(2):291–5.
Estalella I, Rica I, Perez de Nanclares G, et al. Mutations in GCK and HNF-1alpha explain the majority of cases with clinical diagnosis of MODY in Spain. Clin Endocrinol (Oxf). 2007;67(4):538–46.
Tatsi C, Kanaka-Gantenbein C, Vazeou-Gerassimidi A, et al. The spectrum of HNF1A gene mutations in Greek patients with MODY3: relative frequency and identification of seven novel germline mutations. Pediatr Diabetes. 2013;14(7):526–34.
Yorifuji T, Fujimaru R, Hosokawa Y, et al. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes. 2012;13(1):26–32.
Nishigori H, Yamada S, Kohama T, et al. Mutations in the hepatocyte nuclear factor-1 alpha gene (MODY3) are not a major cause of early-onset non-insulin-dependent (type 2) diabetes mellitus in Japanese. J Hum Genet. 1998;43(2):107–10.
Kawakita R, Hosokawa Y, Fujimaru R, et al. Molecular and clinical characterization of glucokinase maturity-onset diabetes of the young (GCK-MODY) in Japanese patients. Diabet Med. 2014;31(11):1357–62.
Ng MC, Cockburn BN, Lindner TH, et al. Molecular genetics of diabetes mellitus in Chinese subjects: identification of mutations in glucokinase and hepatocyte nuclear factor-1alpha genes in patients with early-onset type 2 diabetes mellitus/MODY. Diabet Med. 1999;16(11):956–63.
Xu JY, Chan V, Zhang WY, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in Chinese MODY families: prevalence and functional analysis. Diabetologia. 2002;45(5):744–6.
Xu JY, Dan QH, Chan V, et al. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet. 2005;13(4):422–7.
Lim DM, Huh N, Park KY. Hepatocyte nuclear factor 1-alpha mutation in normal glucose-tolerant subjects and early-onset type 2 diabetic patients. Korean J Intern Med. 2008;23(4):165–9.
Lee HJ, Ahn CW, Kim SJ, et al. Mutation in hepatocyte nuclear factor-1alpha is not a common cause of MODY and early-onset type 2 diabetes in Korea. Acta Diabetol. 2001;38(3):123–7.
Mohan V, Ramachandran A, Snehalatha C, et al. High prevalence of maturity-onset diabetes of the young (MODY) among Indians. Diabetes Care. 1985;8(4):371–4.
Radha V, Ek J, Anuradha S, et al. Identification of novel variants in the hepatocyte nuclear factor-1alpha gene in South Indian patients with maturity onset diabetes of young. J Clin Endocrinol Metab. 2009;94(6):1959–65.
Anuradha S, Radha V, Mohan V. Association of novel variants in the hepatocyte nuclear factor 4A gene with maturity onset diabetes of the young and early onset type 2 diabetes. Clin Genet. 2011;80(6):541–9.
Kanthimathi S, Jahnavi S, Balamurugan K, et al. Glucokinase gene mutations (MODY 2) in Asian Indians. Diabetes Technol Ther. 2014;16(3):180–5.
Chapla A, Mruthyunjaya MD, Asha HS, et al. Maturity onset diabetes of the young in India—a distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf). 2015;82(4):533–42.
Stern E, Strihan C, Potievsky O, et al. Four novel mutations, including the first gross deletion in TCF1, identified in HNF4a, GCK and TCF1 in patients with MODY in Israel. J Pediat Endocrinol Metabol. 2007;20(8):909–22.
Gozlan Y, Tenenbaum A, Shalitin S, et al. The glucokinase mutation p.T206P is common among MODY patients of Jewish Ashkenazi descent. Pediatr Diabetes. 2012;13(6):e14–21.
Woodhouse NJ, Elshafie OT, Al-Mamari AS, et al. Clinically-defined maturity onset diabetes of the young in Omanis: absence of the common Caucasian gene mutations. Sultan Qaboos Univ Med J. 2010;10(1):80–3.
Elbein SC, Teng K, Eddings K, et al. Molecular scanning analysis of hepatocyte nuclear factor 1a (TCF1) gene in typical familial type 2 diabetes in African Americans. Metab Clin Exp. 2000;49(2):280–4.
Furuzawa GK, Giuffrida FM, Oliveira CS, et al. Low prevalence of MODY2 and MODY3 mutations in Brazilian individuals with clinical MODY phenotype. Diabetes Res Clin Pract. 2008;81(3):e12-4.
Mota AJ, Bruggemann S, Costa FF. MODY 2: mutation identification and molecular ancestry in a Brazilian family. Gene. 2013;512(2):486–91.
Weinert LS, Silveiro SP, Giuffrida FM, et al. Three unreported glucokinase (GCK) missense mutations detected in the screening of thirty-two Brazilian kindreds for GCK and HNF1A-MODY. Diabetes Res Clin Pract. 2014;106(2):e44-8.
Dominguez-Lopez A, Miliar-Garcia A, Segura-Kato YX, et al. Mutations in MODY genes are not common cause of early-onset type 2 diabetes in Mexican families. JOP. 2005;6(3):238–45.
Chambers C, Fouts A, Dong F, Colclough K, Wang Z, Batish SD, et al. Characteristics of maturity onset diabetes of the young in a large diabetes center. Pediatr Diab. 2015. doi:10.1111/pedi.12289.
Bennett JT, Vasta V, Zhang M, et al. Molecular genetic testing of patients with monogenic diabetes and hyperinsulinism. Mol Genet Metab. 2015;114(3):451–8.
Pihoker C, Gilliam LK, Ellard S, et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for diabetes in youth. J Clin Endocrinol Metab. 2013;98(10):405–62. The first systematic study of the prevalence of MODY in an unselected pediatric cohort reveals 8% of diabetic children who are antibody negative and C-peptide positive have mutations classified by the authors as pathogenic or likely pathogenic mutations in HNF1A, HNF4A or GCK. Of the 47 mutation carriers, only 3 (6%) had a previous diagnosis, providing evidence that most MODY cases are being missed in the United States. Most were on insulin or metformin, and many were from ethnic groups with high prevalence of type 2 diabetes.
Flannick J, Beer NL, Bick AG, et al. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat Genet. 2013;45(11):1380–5.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–23.
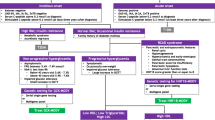
Naylor R, Philipson LH. Who should have genetic testing for maturity-onset diabetes of the young? Clin Endocrinol (Oxf). 2011;75(4):422–6.
Pinelli M, Acquaviva F, Barbetti F, et al. Identification of candidate children for maturity-onset diabetes of the young type 2 (MODY2) gene testing: a seven-item clinical flowchart (7-iF). PLoS One. 2013;8(11):e79933.
Carroll RW, Murphy R. Monogenic diabetes: a diagnostic algorithm for clinicians. Genes (Basel). 2013;4(4):522–35.
Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34(8):1878–84.
Stride A, Ellard S, Clark P, et al. Beta-cell dysfunction, insulin sensitivity, and glycosuria precede diabetes in hepatocyte nuclear factor-1alpha mutation carriers. Diabetes Care. 2005;28(7):1751–6.
Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4(4):e118.
Hamilton AJ, Bingham C, McDonald TJ, et al. The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a beta cell phenotype. J Med Genet. 2014;51(3):165–9.
Bellanne-Chantelot C, Clauin S, Chauveau D, et al. Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54(11):3126–32.
Dubois-Laforgue D, Bellanne-Chantelot C, Subra JF, et al. Pectus excavatum is part of the clinical spectrum of HNF1B MODY5. Diabetes Care. 2014;37(4):e72–3.
Spegel P, Ekholm E, Tuomi T, et al. Metabolite profiling reveals normal metabolic control in carriers of mutations in the glucokinase gene (MODY2). Diabetes. 2013;62(2):653–61.
Steele AM, Wensley KJ, Ellard S, et al. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS One. 2013;8(6):e65326.
Fendler W, Borowiec M, Antosik K, et al. HDL cholesterol as a diagnostic tool for clinical differentiation of GCK-MODY from HNF1A-MODY and type 1 diabetes in children and young adults. Clin Endocrinol (Oxf). 2011;75(3):321–7.
Ridker PM, Pare G, Parker A, et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet. 2008;82(5):1185–92.
Reiner AP, Barber MJ, Guan Y, et al. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1 alpha are associated with C-reactive protein. Am J Hum Genet. 2008;82(5):1193–201.
Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011;54(11):2801–10.
Owen KR, Thanabalasingham G, James TJ, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010;33(9):1919–24.
Bellanne-Chantelot C, Coste J, Ciangura C, Fonfrède M, Saint-Martin C, Bouché C, et al. High-sensitivity C-reactive protein does not improve the differential diagnosis of HNF1A-MODY and familial young-onset type 2 diabetes: a grey zone analysis. Diabetes Metab. 2015. doi:10.1016/j.diabet.2015.02.001.
Thanabalasingham G, Huffman JE, Kattla JJ, et al. Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes. 2013;62(4):1329–37.
Bacon S, Kyithar MP, Schmid J, et al. Circulating CD36 is reduced in HNF1A-MODY carriers. PLoS One. 2013;8(9):e74577.
Nowak N, Szopa M, Thanabalasingham G, et al. Cystatin C is not a good candidate biomarker for HNF1A-MODY. Acta Diabetol. 2013;50(5):815–20.
Nowak N, Hohendorff J, Solecka I, Szopa M, Skupien J, Kiec-Wilk B, et al. Circulating ghrelin level is higher in HNF1A-MODY and GCK-MODY than in polygenic forms of diabetes mellitus. Endocrine. 2015. doi:10.1007/s12020-015-0627-5.
Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56(9):1958–63. First publication of targeted next generation sequencing test for monogenic diabetes.
Bonnefond A, Philippe J, Durand E, et al. Highly sensitive diagnosis of 43 monogenic forms of diabetes or obesity through one-step PCR-based enrichment in combination with next-generation sequencing. Diabetes Care. 2014;37(2):460–7.
Gao R, Liu Y, Gjesing AP, et al. Evaluation of a target region capture sequencing platform using monogenic diabetes as a study-model. BMC Genet. 2014;15:13,2156-15-13.
Johansson S, Irgens H, Chudasama KK, et al. Exome sequencing and genetic testing for MODY. PLoS One. 2012;7(5):e38050.
Dusatkova P, Fang M, Pruhova S, et al. Lessons from whole-exome sequencing in MODYX families. Diabetes Res Clin Pract. 2014;104(3):e72–4.
Stein SA, Maloney KL, Pollin TI. Genetic counseling for diabetes mellitus. Curr Genet Med Rep. 2014;2(2):56–67. Suggested approach for evaluating a family history for the likelihood of MODY vs. polygenic diabetes.
Acknowledgments
Jeffrey W. Kleinberger and Toni I. Pollin are supported by NIH U01 HG007775, which provides funding for the Personalized Diabetes Medicine Program discussed and is a component of the IGNITE (Implementing Genomics in Practice) Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jeffrey W. Kleinberger and Toni I. Pollin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
This article is part of the Topical Collection on Diabetes Epidemiology
Rights and permissions
About this article
Cite this article
Kleinberger, J.W., Pollin, T.I. Undiagnosed MODY: Time for Action. Curr Diab Rep 15, 110 (2015). https://doi.org/10.1007/s11892-015-0681-7
Published:
DOI: https://doi.org/10.1007/s11892-015-0681-7