Abstract
The eastern deciduous forest is a mix of arbuscular (AM) and ectomycorrhizal (EM) trees, but land use legacies have increased the abundance of AM trees like Acer spp. (maple). Although these legacies have not changed the abundance of some EM trees like Betula spp. (birch), EM conifers like Tsuga canadensis (hemlock), and Pinus strobus (pine) have declined. We used a soil bioassay to investigate if the microbial community near EM birch (birch soil) contains a greater abundance and diversity of EM fungal propagules compatible with T. canadensis and P. strobus compared to the community associated with the surrounding AM-dominated secondary forest matrix (maple soil). We also tested the effectiveness of inoculation with soil from a nearby EM-dominated old-growth forest as a restoration tool to reintroduce EM fungi into secondary forest soils. Finally, we examined how seedling growth responded to EM fungi associated with each treatment. Seedlings grown with birch soil were colonized by EM fungi mostly absent from the surrounding maple forest. Hemlock seedlings grown with birch soil grew larger than hemlock seedlings grown with maple soil, but pine seedling growth did not differ with soil treatment. The addition of old-growth soil inoculum increased hemlock and pine growth in both soils. Our results found that EM trees are associated with beneficial EM fungi that are mostly absent from the surrounding AM-dominated secondary forest, but inoculation with old-growth soil is effective in promoting the growth of seedlings by reintroducing native EM fungi to the AM-dominated forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The North American eastern deciduous forest is comprised of a mix of arbuscular mycorrhizal (AM) and ectomycorrhizal (EM) forest types that can form patches in the landscape (Frelich et al. 1993; Phillips et al. 2013). While AM fungi colonize most plant genera including herbaceous plants and the tree genera Acer (maple), Fraxinus (ash), and Prunus (cherry), EM fungi colonize a narrower range of host plants such as Betula (birch), Fagus (beech), Pinus (pine), Tsuga (hemlock), and Quercus (oak) (Brundrett 2009; Smith and Read 2010; Brundrett and Tedersoo 2020). Anthropogenic impacts such as agricultural land use, logging, and nitrogen deposition, as well as the introductions of hemlock woolly adelgid and white pine blister rust have decreased the abundance of EM conifers such as Tsuga canadensis (eastern hemlock) and Pinus strobus (eastern white pine) in the eastern deciduous forest (Braun 1950; Whitney 1990; Hummer 2000; Abrams 2001; Ellison et al. 2005). There are now extensive areas of forest dominated by AM broadleaf trees such as maple, with fewer EM conifer trees like hemlock and pine (Abrams 1998; Dyer 2001, 2006; Phillips et al. 2013; Thompson et al. 2013; Jo et al. 2019), while the abundance of some EM trees like birch has not changed appreciably (Whitney 1990; Flinn and Marks 2007).
Hemlock and pine provide important structural heterogeneity in forests otherwise comprised exclusively of deciduous broadleaf trees (Abrams et al. 1995; Yamasaki et al. 2000), and declines in their abundance have had broad ecological effects (Abrams 2001; Snyder et al. 2002; Ellison et al. 2005). The reintroduction of hemlock and pine into forests is desirable to restore ecosystem function and habitat heterogeneity of eastern forests (Vellend et al. 2007; Burton and Macdonald 2011), but factoring in their obligate associations with EM fungi is critical for restoration to be successful (Policelli et al. 2020).
While AM fungi are usually not limiting in terrestrial ecosystems such as agricultural fields and forests (Rillig 2004; Brundrett 2009; Gottshall et al. 2017), EM fungi may not be as widely distributed (Peay et al. 2010, 2012). As a result, a greater abundance and diversity of EM fungal propagules beneficial for seedling establishment can be found in soils associated with existing EM vegetation (Borchers and Perry 1990; Horton et al. 1999; Nara 2006a, b) which can drive positive plant-soil feedbacks and the formation of patches dominated by EM trees (McGuire 2007; Bennett et al. 2017; Montesinos‐Navarro et al. 2019; Eagar et al. 2020).
Since EM fungi are often dispersal limited (Nuñez et al. 2009; Peay et al. 2010, 2012; Galante et al. 2011), AM-dominated patches may have a relatively low availability of EM fungal propagules. However, scattered individuals or patches of EM plants have been documented to support EM fungal communities that may influence EM inoculum and seedling establishment (van der Heijden et al. 1998; Baxter and Dighton 2001; Jonsson et al. 2001; Dickie et al. 2002; Thiet and Boerner 2007; Matsuda et al. 2013). To the best of our knowledge, no previous studies have examined the role of isolated EM patches on EM fungal inoculum within undisturbed eastern deciduous forests.
In areas lacking EM fungi, inoculation with soil from a reference site, such as a local EM-dominated forest (Policelli et al. 2020), can transfer EM fungal propagules and improve the growth, nutrient status, and survival of seedlings in restoration projects (Amaranthus and Perry 1987; Perry et al. 1987; Cortese and Bunn 2017). Further, inoculation usually selects for resistant propagules which represent a subset of the whole EM fungal community but can be critical for seedling establishment (Taylor and Bruns 1999; Baar et al. 1999; Ashkannejhad and Horton 2006; Izzo et al. 2006).
For our study, we conducted growth chamber experiments to investigate how hemlock and pine seedling establishment are influenced by their associations with EM fungi in AM-dominated secondary forest soils. The purpose of Experiment 1 was to determine whether the soil microbial community associated with scattered EM birch (Betula lenta, Betulaceae) trees were represented by a greater abundance and diversity of EM fungi compatible with eastern hemlock (Tsuga canadensis, Pinaceae) and white pine (Pinus strobus, Pinaceae) seedlings compared to the soil microbial community associated with the surrounding AM maple (Acer saccharum and A. rubrum, Sapindaceae) dominated forest. We also investigated how the soil microbial communities associated with EM birch and AM maple influenced the growth of pine and hemlock seedlings. Individual and small groups of EM Betula lenta (birch) trees have established in isolated locations within a matrix of otherwise dominant AM Acer spp. (maple) forest patches and represent the most abundant and widely distributed EM tree in the secondary AM-dominated forest. The purpose of Experiment 2 was to investigate whether soil inoculum collected from a nearby old-growth forest dominated by the EM trees Tsuga canadensis, Betula lenta, and Quercus spp. could be used as a restoration tool to reintroduce native EM fungi and subsequently enhance the growth of hemlock and pine seedlings grown in soils from the secondary AM-dominated forest.
The objectives of our study were to (1) determine under growth chamber conditions if the soil microbial community associated with EM birch trees results in greater EM percent colonization, EM root tip biomass, and EM fungal richness of pine and hemlock seedlings compared to the soil microbial community associated with AM maple, (2) determine under growth chamber conditions if the soil microbial community associated with EM birch positively influences the growth of pine and hemlock seedlings compared to the soil microbial community associated with AM maple, and (3) test under growth chamber conditions how the addition of fungal inoculum from EM-dominated old-growth forest soils influences mycorrhizal colonization and growth of pine and hemlock seedlings grown in soils from an AM-dominated secondary forest.
Materials and methods
Site description
Our study took place at the Mianus River Gorge (MRG) in Westchester County, NY. It is part of a 303-ha nature preserve that features over 200 ha of c. 80-year-old secondary maple-dominated forest growing on abandoned agricultural fields as well as 100 ha of old-growth hemlock-hardwood forest in a steep river gorge (Weckel et al. 2006). We estimated stand-level overstory composition using the point-centered quarter method (Mueller-Dombois and Ellenberg 1974) from three secondary maple-dominated forest stands as well as the old-growth EM-dominated forest. We identified Betula lenta (birch) as the dominant EM tree and two species of maple, A. rubrum and A. saccharum (shown simply as “maple” for the rest of this paper) as the dominant AM tree to be used for our study (Table 1).
Focal tree selection
We selected focal birch trees that were at least 15 m from any overstory EM tree (DBH ≥ 10 cm) in Pinaceae (e.g., Pinus and Tsuga), and we selected focal maple trees that were at least 15 m from any overstory EM trees (DBH ≥ 10 cm). We chose this distance to avoid contact with the lateral extent of EM tree root systems and associated mycelial networks (Lilleskov et al. 2004; Dickie and Reich 2005). There was one 10.1 cm birch tree 14.1 m from one maple focal tree (Table 1). Using these criteria, we were able to select 8 maple and 8 birch trees for the collection of soil inoculum. At each tree, we then established 15 m radius plots and measured diameter at breast height (DBH) for all trees and saplings ≥ 137 cm tall (Table 1).
Experiment 1: collection of soils near EM birch and AM maple from secondary forest
Soils were collected from the base of eight Betula lenta (birch) and eight Acer spp. (maple) from a maple-dominated, second-growth forest at the Mianus River Gorge Preserve, Westchester County, NY, in October 2019. From each birch and maple tree, 8 L of soil were collected 2 m from the center of the bole at the north and south cardinal directions to enhance the collection of soil microbes associated with the roots of each focal tree. Soils were excavated from areas approximately 15 cm deep by 30 cm in diameter using a shovel sterilized with 10% bleach between collections. Rocks and woody debris were discarded on site. Soils were composited by treatment (EM birch or AM maple) to capture the average soil inoculum potential for soils from the two mycorrhizal trees (Allen et al. 2021) and were stored at 4 °C for 1 week.
Experiment 2: collection of soil from EM-dominated old-growth forest
EM-dominated old-growth forest soil inoculum (old growth) was collected from an adjacent old-growth forest dominated by EM trees Betula lenta, Tsuga canadensis, and Quercus spp. This forest was adjacent to the maple-dominated secondary forest (Weckel et al. 2006). Old-growth soil inoculum was collected in a similar manner as birch/maple soil but only from the base of four healthy Tsuga canadensis and four healthy Quercus spp. Old-growth soils were also composited and stored at 4 °C for 1 week.
Experiments 1 and 2: soil processing
All soils were transported to SUNY ESF, Syracuse, NY, homogenized, passed through a 4.75 mm mesh (# 4 US Standard), sieve to remove pebbles and organic debris, and then dried for 3 weeks at room temperature (20 °C) to select for resistant EM fungal propagules (Baar et al. 1999). Subsets of each soil type (birch, maple, and old growth inoculum) were sterilized (autoclaved once at 121 °C for 45 min, allowed to rest overnight, and autoclaved again under the same conditions to kill spore germinants). Live and sterilized birch and maple soils (secondary forest soils) were mixed with sterilized sand in a 3:1 ratio of soil:sand (v/v), and then live birch and maple soil mixes were combined in a 1:1 (v/v) ratio with autoclaved soil from the opposing treatment. We did this to control for any physical and chemical differences to be able to isolate effects of the birch and maple soil microbial community on plant growth response (hereafter referred to as “birch soil” and “maple soil”). Sterile controls were a 1:1 (v/v) mix of autoclaved birch soil/sand mixture and maple soil/sand. One representative soil sample was analyzed for pH, carbon, nitrogen, and phosphorus at the SUNY ESF Forest Soils Laboratory. Soil pH was measured by mixing a 1:1 (v/v) slurry of soil and dH2O and then measuring with a Corning 445 pH meter. Total carbon and nitrogen were analyzed with a FlashEA® C/N analyzer, and phosphorous was measured using the Bray-1 extraction method. The secondary forest soils had a pH of 6.12, with 0.21% total nitrogen, 0.001% phosphorus, and 3.55% carbon by volume.
Seedling bioassay: hemlock and pine growth and EM colonization in secondary forest soils
Birch, maple, and sterile soils (secondary forest soils) were used for a growth chamber bioassay, utilizing a full-factorial design with a sterile control conducted simultaneously for Experiment 1 and Experiment 2. Tsuga canadensis (hemlock) and P. strobus (pine) seeds (Sheffield Seed©, Locke, NY) were surface sterilized in 3% H2O2 for 10 min, rinsed in distilled H2O, and cold-stratified at 4 °C for 75 days. Hemlock and pine seeds were sown in SC-10 Conetainers© (Steuwe and Sons©) that were soaked in 10% bleach for 24 h prior to sowing. For the birch + old growth and maple + old growth treatments, each tube was filled with the respective (birch or maple) secondary forest soil followed by a layer of old-growth forest soil inoculum. Each tube was then topped off with a thin layer of the respective secondary forest soil for a final ratio of 1:9 (v/v) old-growth soil inoculum to secondary forest soil. To control for potential chemical effects on seedling growth, birch and maple soil treatments, and sterile controls each received 1:9 (v/v) of autoclaved old-growth soil in the same manner as previously stated. Between 3 and 5 seeds were sown in each tube at an approximate depth of 5 mm, allowed to germinate, and were then thinned to one seedling per tube after 1 month. We planted a total of 22 replicate tubes per treatment combination for each species. Seedlings were grown in a growth chamber at 20 °C at an average light intensity of 500 μmol m−2 s−1 and photoperiod of 16:8 h light:dark for 4 months and racks were randomized once every 4 weeks. Due to the shutdown of the SUNY ESF campus during the onset of the COVID-19 pandemic in March 2020, seedlings were moved to an outdoor greenhouse without additional lighting or climate control and grown for 2 months. Seedlings were later returned to the original growth chamber in June 2020 and grown under the previous light and temperature conditions for an additional 3 months before harvest in September 2020. The seedlings grew for a total of nine months and were watered as needed throughout the experiment.
Seedling harvest
Upon harvest, each seedling root system was gently washed under tap water over a 1-mm mesh (#18 US Standard) sieve to remove soil. The shoot was removed at the root collar, and the initial wet weight of the root system was recorded. Following the assessment of mycorrhizal colonization, the remaining wet root system from each seedling was re-weighed, dried at 60 °C for 48 h, and then weighed again. The dry weight of each entire root system was then estimated by using linear regression coefficients from the relationship of post-assessment wet and dry weights with the initial wet weight measurement. Shoots were placed in envelopes, dried at 60 °C for 48 h, and then weighed.
Morphological examination of root tips
To assess the percent mycorrhizal colonization and EM fungal richness of seedlings, root systems were examined under a stereomicroscope (Nikon® 645) at 40 × magnification. Each root tip was counted from seedlings with small root systems (0–200 root tips), while large root systems with many root tips were subsampled by cutting them into 1–3 cm sections, homogenizing in a gridded petri dish, and then selecting root sections by generating random coordinates until a minimum of 150 live root tips were examined per seedling. Using these methods, between 63 and 589 individual root tips were counted per seedling. EM root tips were identified as swollen and turgid relative to uncolonized roots; cross sections of questionable root tips were stained in trypan blue and examined under a compound microscope (Nikon® E600) at 400 × magnification for the presence of a mantle and Hartig net. EM root tips were counted and separated into unique morphotypes based on color, branching, and mantle characteristics (Agerer 1987-2002). One control pine seedling had apparent EM contamination based on an EM morphotype that did not amplify, but the seedling was not included in any subsequent analyses. All root tips from each unique morphotype were removed from the root system, placed in dH20 in 1.5-ml Eppendorf tubes, lyophilized (LabConco©), and then weighed on a microbalance (Mettler-Toledo©) to measure EM root tip biomass. Total EM biomass for all subsampled roots was calculated by estimating the relationship between dry root biomass and the number of root tips for non-subsampled seedlings via linear regression. Biomass measurements for each seedling were then scaled by multiplying the EM biomass by the proportion of root tips counted divided by the total number of root tips estimated by linear regression coefficients.
Molecular identification of EM root tips
DNA was extracted from 1–5 lyophilized root tips of each unique morphotype per seedling following the CTAB method (Gardes and Bruns 1993). Polymerase chain reaction (PCR) with the ITS1-F and ITS4 primers was used to amplify the nuclear ribosomal internal transcribed spacer (ITS) region for each morphotype isolated from each seedling (White et al. 1990; Gardes and Bruns 1993). Restriction fragment length polymorphisms (RFLP) were then generated from each amplicon using HinfI and DpnII restriction enzymes (New England Biolabs©, Ipswich, MA) and visualized on 3% agarose gels. Unique RFLP types based on the two restriction digests were considered operational taxonomic units (OTU) and used as proxies for species (Kårén et al. 1997; Horton 2002) and calculation of species richness (Gardes and Bruns 1996). For each unique RFLP type, the ITS region was again amplified using ITS1-F and ITS4 primers, cleaned using a Qiaquick PCR purification kit (Qiagen©, Germantown, MD), and sequenced (Eurofins Genomics©, Lousville, KY). Sequences were visually examined for quality using Chromas© 2.6.6 software and then compared to sequences in Genbank (http://blast.ncbi.nlm.nih.gov/) for taxonomic assignments based on sequence similarities of reference taxa in the database. Sequences that matched ≥ 97% across the ITS region were considered the same species (Schoch et al. 2012). Sequences that were between 97 and 93% similar across the ITS region were considered the same genus. Unknown sequences in our study identified to a genus were placed into an alignment by genus using SeaView 4 and were placed within a species at ≥ 97% base pair similarity. Unique species within a genus that were not matched to a named species were given numerical identifiers (e.g., Tuber sp. 1, Tuber sp. 2).
Statistical analysis
All statistical analyses were conducted in the RStudio statistical environment using R version 4.0.2 (R Core Team 2020). Separate statistical tests were conducted for hemlock and pine seedling datasets, but data from Experiment 1 and Experiment 2 were analyzed with the same tests for each tree species. Total biomass, root:shoot ratio, and foliar nutrition from hemlock and pine, as well as EM biomass and percent colonization of pine seedlings, were conducted using two-factor ANOVA. Tukey HSD pairwise comparisons of significant ANOVA (p < 0.05) were conducted using the emmeans package. Data were log transformed as needed to fulfill assumptions of normality and homogeneity of variance. Hemlock EM biomass and percent colonization, as well as pine and hemlock OTU richness, did not meet assumptions of ANOVA after transformation. This was because the maple soil treatment resulted in EM colonization of seedlings that was too low to detect a treatment response. The maple soil treatment group was subsequently omitted and the remaining groups (birch, maple + old growth, and birch + old growth) were compared using nonparametric Kruskal–Wallis with Wilcoxon rank-sum pairwise tests and Bonferroni p-value adjustments.
Results
Molecular identification of EM fungi
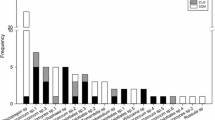
In total, we identified 12 EM fungal OTUs from a total of 34 surviving hemlock and 98 surviving pine seedlings, on which Cenococcum geophilum was the most frequently encountered (19 hemlock and 42 pine), followed by Hyaloscypha bicolor (7 hemlock and 10 pine), and then Tuber arnoldianum (1 hemlock and 9 pine; Table 2). Despite being the most frequent, C. geophilum was only present on seedlings grown with the birch soil microbial community (birch soil), or seedlings with old-growth soil inoculum added. Tuber arnoldianum was the only EM fungal OTU identified from seedlings grown with the maple soil microbial community (maple soil) and was also found in all other treatments except the sterile control (Table 2). Genbank accession numbers for all identified EM fungi can be found in Table 5 in the Appendix.
Experiment 1: influence of scattered EM birch on EM colonization and seedling growth
Hemlock seedlings
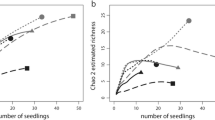
Hemlock seedlings grown with maple soil had extremely sparse percent EM colonization as determined by visual examination of roots as well as low EM biomass which yielded no PCR products (Fig. 1). In contrast, hemlock seedlings grown with birch soil were all clearly mycorrhizal-based on visual examination of the roots and were colonized primarily by Cenococcum geophilum. Hemlock seedlings grown with birch soil had significantly greater shoot and total biomass, but not root biomass compared to seedlings grown with maple soil. Hemlock seedlings grown with maple soil had a greater root:shoot ratio than seedlings grown with birch soil (Table 3).
EM fungal operational taxonomic unit (OTU) richness (top), EM biomass (middle), and percent EM colonization (bottom) of Tsuga canadensis (hemlock, left) and Pinus strobus (pine, right) seedlings grown with the soil microbial community associated with EM Betula lenta (birch) and AM Acer spp. (maple) from the surrounding AM-dominated forest. Some seedlings also received an addition of EM-dominated old-growth forest soil inoculum (+ OG). Pine EM biomass and percent EM colonization were compared using two-factor ANOVA, and letters indicate significant Tukey HSD pairwise comparisons (p < 0.05). All other analyses were conducted with Kruskal–Wallis tests with the maple treatment omitted due to a lack of ectomycorrhizal response (*). No significant differences were detected between any of the remaining treatment groups (p ≥ 0.05)
Pine seedlings
Pine seedlings grown with maple soil had sparse percent EM colonization and low EM biomass compared to birch soil (Fig. 1). Tuber arnoldianum was the only EM fungal OTU identified from a single pine seedling grown with maple soil, while seedlings grown with birch soil were dominated by Cenococcum geophilum (Fig. 1, Table 2). We detected no significant differences in shoot, root, or total biomass between pine seedlings grown with maple versus birch soil, or the sterile control soil. We also found no difference in root:shoot ratio between treatments (Table 4).
Experiment 2: Influence of old-growth soil inoculation on EM colonization and seedling growth
Hemlock seedlings
Addition of old-growth soil increased the percent EM colonization, EM root tip biomass, and EM fungal OTU richness on hemlock seedlings grown with maple soil, but not birch soil. This resulted in similar EM colonization of all mycorrhizal treatments (birch, maple + old growth, birch + old growth) except for maple soil alone (Fig. 1). Addition of old-growth soil inoculum to birch soil resulted in the detection of new EM fungal OTUs not present on seedlings grown without old growth soil, including Tuber canaliculatum (Table 2). Addition of old-growth soil inoculum increased the shoot and total biomass of hemlock seedlings grown with both maple and birch soil, as well as the EM root tip biomass of hemlock grown with birch soil. Seedlings grown with old-growth soil inoculum grew about twice as large as seedlings grown without old-growth soil, and seedlings grown in maple soil with old-growth soil inoculum added exhibited comparable root:shoot ratios to other ectomycorrhizal treatments (birch, birch + old growth) (Table 3).
Pine seedlings
Addition of old-growth soil inoculum also increased the percent EM colonization, EM root tip biomass, and EM fungal OTU richness on pine seedlings grown with maple, but not birch soil. This resulted in similar EM colonization of all treatments (birch, maple + old growth, birch + old growth) except for maple soil alone (Fig. 1). As with hemlock, addition of old-growth soil inoculum to seedlings grown with birch soil resulted in the detection of EM fungal OTUs not present on seedlings grown without old-growth soil including three additional Tuber spp. and Suillus salmonicolor (Table 2). Addition of old-growth soil inoculum increased the root, shoot, and total biomass of seedlings grown with both maple and birch soil. Seedlings grown with the addition of old-growth soil inoculum grew about twice as large as seedlings grown without old-growth soil, but there were no effects on root:shoot ratio (Table 4).
Discussion
Ectomycorrhizal Betula lenta (birch) are associated with a greater richness and abundance of EM fungi available to pine and hemlock seedlings compared to the surrounding maple forest. This shows that in AM-dominated forests such as those dominated by maple, scattered EM trees influence EM fungal communities similar to pioneering EM hardwoods (Borchers and Perry 1990; Horton et al. 1999; Kennedy et al. 2012) in the Pacific Northwest and isolated EM oak trees in an AM-dominated Japanese cypress forest (Matsuda et al. 2013). In our study, resistant EM fungal propagule communities in soils associated with birch trees were dominated primarily by Cenococcum geophilum, Hyaloscypha bicolor, and Tomentella sublilacina, while Tuber arnoldianum was detected from both maple and birch soil EM fungal propagule communities. These fungi are all known to produce resistant propagules such as sclerotia and spores that are sources of EM fungi beneficial for initial seedling establishment following disturbance (Baar et al. 1999; Izzo et al. 2006; Bonito et al. 2012; Glassman et al. 2015). However, these fungi are often less functional in undisturbed forests in which active EM fungal mycelium is the primary inoculum source (Cline et al. 2005; Grove et al. 2019). As a result, these fungi are generally more abundant on seedlings grown in bioassays compared to those grown in field conditions (Baar et al. 1999; Taylor and Bruns 1999; Dulmer et al. 2014). While C. geophilum inoculum (microsclerotia) is considered ubiquitous (Trappe 1962) and has been reported far from established EM trees in mixed AM-EM forests (Dickie et al. 2002), it was not detected in maple soils in our study indicating a paucity of this common taxon in areas without EM trees in our area. This may be due to C. geophilum reproducing exclusively via asexual microsclerotia produced belowground that may not disperse well beyond its established ramets. Pooling replicate soil samples and further mixing of live and sterilized soils in our experiment likely increased the dominance of C. geophilum, which can reproduce from an individual microsclerotium (Trappe 1962). Additionally, soil mixing could have discriminated against other EM fungal taxa through dilution of spore concentrations below levels necessary to form ectomycorrhizae (Ashkannejhad and Horton 2006; Bruns et al. 2009), which could have prevented their detection in our study. We found that EM fungal propagules associated with scattered birch trees resulted in increased growth and a lower root:shoot ratio for hemlock seedlings. This is likely due to enhanced nutrient acquisition by EM-colonized seedlings which resulted in proportional decreases in belowground carbon allocation and increased shoot growth (Bloom et al. 1985). In contrast, pine seedling growth was less responsive to EM fungal propagules associated with birch trees compared to the surrounding maple forest. One possible explanation for the discordant growth responses of pine and hemlock is due to differences in seed size between the two species. Hemlock seeds are about 10% the size of pine seeds (Service and Bonner 2008) and contain lower levels of endosperm nutrients. First-year hemlock seedlings produce small, shallow root systems (Godman and Lancaster 1990) which may be more dependent on colonization by EM fungi for establishment than larger-seeded pines (Abuzinadah et al. 1986; Holste et al. 2017).
The addition of soil inoculum collected from a local EM-dominated old-growth forest increased the amount of EM fungal colonization and EM fungal richness of both pine and hemlock seedlings grown with maple soil, but not birch soil. The addition of old-growth soil inoculum resulted in colonization by Tuber canaliculatum, T. separans, and the pine-specialist Suillus salmonicolor, which were not detected on seedlings that did not receive old-growth soil inoculum. The presence of these fungi suggests that the old-growth forest supports EM fungal propagules that are unable to naturally disperse (Peay et al. 2010), or if able to disperse cannot lie dormant in the secondary forest. These additional taxa may be important for EM seedling establishment since the addition of old-growth soil inoculum corresponded to considerable increases in the growth of pine and hemlock seedlings relative to growth in birch or maple soil alone.
Soil inoculation is context dependent (Hoeksema et al. 2010), in which some studies show positive effects on the formation of mycorrhizae of planted seedlings (Amaranthus and Perry 1987; Cortese and Bunn 2017) while others show no measurable effect (Sýkorová et al. 2016; Grove et al. 2019). The introduction of new EM fungal taxa and increased seedling growth response suggest inoculation can be effective in AM-dominated forests with low EM fungal inoculum (Policelli et al. 2020). The dominance of AM trees could be the result of pervasive land use legacies (Flinn and Marks 2007) that limit the natural recolonization of EM trees (Rogers 1978; Ribbens et al. 1994; McEuen and Curran 2004) in conjunction with dispersal limitations of EM fungi (Peay et al. 2010). These effects may not be consistent among all EM trees, where birch may be well-adapted to recolonization due to broad seed dispersal (Matlack 1989) and the ability of seedlings to establish in sites with low EM fungal inoculum (Collier and Bidartondo 2009). The apparent lack of EM fungal propagules compatible with hemlock and pine within the maple-dominated forest demonstrates that the low EM fungal inoculum in some secondary forests may preclude the successful establishment of EM conifers in otherwise favorable sites. This, in conjunction with the ability of maple to produce large quantities of seed capable of establishing in undisturbed leaf litter (Frelich et al. 1993; Abrams 1998; Southgate and Thompson 2014), may contribute to the continued dominance of AM maple while concomitantly limiting the natural recolonization of EM conifers in AM-dominated secondary forests. Planting EM conifer seedlings with soil inoculum from a reference site, or in the vicinity of existing EM trees are two restoration strategies that can ensure access to critical EM fungi and support their establishment.
Conclusion
Secondary maple-dominated forests appear to lack sufficient EM fungal propagules to support pine and hemlock seedlings except in the vicinity of scattered EM trees. Due to effects on growth, seedling establishment may be limited by access to EM fungi in these locations. However, the addition of soil inoculum from an old-growth forest resulted in greater overall EM fungal diversity as well as seedling growth. The reduction of EM fungal propagules in secondary forest soils may be yet another legacy of pervasive land use in the eastern deciduous forest, with implications for the natural regeneration, as well as the restoration of EM conifer trees following agricultural land abandonment.
Data availability
The data that support the findings of this study are available from the corresponding author, AC, upon reasonable request.
References
Abrams MD (2001) Eastern white pine versatility in the presettlement forest. Bioscience 51:967–979. https://doi.org/10.1641/0006-3568(2001)051[0967:EWPVIT]2.0.CO;2
Abrams MD (1998) The Red Maple Paradox. Bioscience 48:355–364. https://doi.org/10.2307/1313374
Abrams MD, Orwig DA, Demeo TE (1995) Dendroecological analysis of successional dynamics for a presettlement- origin white-pine-mixed-oak forest in the southern Appalachians, USA. J Ecol 83:123–133. https://doi.org/10.2307/2261156
Abuzinadah R, Finlay R, Read D (1986) The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. New Phytol 103:495–506. https://doi.org/10.1111/J.1469-8137.1986.TB02887.X
Agerer R (1987–2002) Colour Atlas of Ectomycorrhizae. Einhorn Verlag, Schwabisch Gmünd
Allen WJ, Sapsford SJ, Dickie IA (2021) Soil sample pooling generates no consistent inference bias: a meta-analysis of 71 plant–soil feedback experiments. New Phytol 231:1308–1315. https://doi.org/10.1111/nph.17455
Amaranthus MP, Perry DA (1987) Effect of soil transfer on ectomycorrhiza formation and the survival and growth of conifer seedlings on old, nonreforested clear-cuts. Can J Res 17:944–950. https://doi.org/10.1139/x87-147
Ashkannejhad S, Horton TR (2006) Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytol 169:345–354. https://doi.org/10.1111/j.1469-8137.2005.01593.x
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytol 143:409–418. https://doi.org/10.1046/j.1469-8137.1999.00452.x
Baxter JW, Dighton J (2001) Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host–symbiont culture conditions. New Phytol 152:139–149. https://doi.org/10.1046/j.0028-646x.2001.00245.x
Bennett JA, Maherali H, Reinhart KO et al (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355:181–184. https://doi.org/10.1126/science.aai8212
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants-an economic analogy. Annu Rev Ecol Syst 16:363–392. https://doi.org/10.1146/annurev.es.16.110185.002051
Bonito G, Smith ME, Brenneman T, Vilgalys R (2012) Assessing ectomycorrhizal fungal spore banks of truffle producing soils with pecan seedling trap-plants. Plant Soil 356:357–366. https://doi.org/10.1007/s11104-012-1127-5
Borchers SL, Perry DA (1990) Growth and ectomycorrhiza formation of Douglas-fir seedlings grown in soils collected at different distances from pioneering hardwoods in southwest Oregon clear-cuts. Can J Res 20:712–721. https://doi.org/10.1139/x90-094
Braun EL (1950) Deciduous Forests of Eastern North America. Blackburn Press, Caldwell
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brundrett MC, Tedersoo L (2020) Resolving the mycorrhizal status of important northern hemisphere trees. Plant Soil 454:3–34. https://doi.org/10.1007/s11104-020-04627-9
Bruns TD, Peay KG, Boynton PJ et al (2009) Inoculum potential of Rhizopogon spores increases with time over the first 4 yr of a 99-yr spore burial experiment. New Phytol 181:463–470. https://doi.org/10.1111/j.1469-8137.2008.02652.x
Burton P, Macdonald S (2011) The restorative imperative: challenges, objectives and approaches to restoring naturalness in forests. Silva Fenn 45:843–863. https://doi.org/10.14214/sf.74
Cline ET, Ammirati JF, Edmonds RL (2005) Does proximity to mature trees influence ectomycorrhizal fungus communities of Douglas-fir seedlings? New Phytol 166:993–1009. https://doi.org/10.1111/j.1469-8137.2005.01387.x
Collier FA, Bidartondo MI (2009) Waiting for fungi: the ectomycorrhizal invasion of lowland heathlands. J Ecol 97:950–963. https://doi.org/10.1111/j.1365-2745.2009.01544.x
Cortese AM, Bunn RA (2017) Availability and function of arbuscular mycorrhizal and ectomycorrhizal fungi during revegetation of dewatered reservoirs left after dam removal: mycorrhizal fungi in dewatered reservoirs. Restor Ecol 25:63–71. https://doi.org/10.1111/rec.12406
Dickie IA, Koide RT, Steiner KC (2002) Influences of established trees on mycorrhizas, nutrition, and growth of Quercus rubra seedlings. Ecol Monogr 72:505–521. https://doi.org/10.1890/0012-9615(2002)072[0505:IOETOM]2.0.CO;2
Dickie IA, Reich PB (2005) Ectomycorrhizal fungal communities at forest edges. J Ecol 93:244–255. https://doi.org/10.1111/j.1365-2745.2005.00977.x
Dulmer KM, LeDuc SD, Horton TR (2014) Ectomycorrhizal inoculum potential of northeastern US forest soils for American chestnut restoration: results from field and laboratory bioassays. Mycorrhiza 24:65–74. https://doi.org/10.1007/s00572-013-0514-y
Dyer JM (2001) Using witness trees to assess forest change in southeastern Ohio. Can J Res 31:1708–1718. https://doi.org/10.1139/cjfr-31-10-1708
Dyer JM (2006) Revisiting the Deciduous Forests of Eastern North America. Bioscience 56:341–352. https://doi.org/10.1641/0006-3568(2006)56[341:RTDFOE]2.0.CO;2
Eagar AC, Cosgrove CR, Kershner MW, Blackwood CB (2020) Dominant community mycorrhizal types influence local spatial structure between adult and juvenile temperate forest tree communities. Funct Ecol 34:2571–2583. https://doi.org/10.1111/1365-2435.13674
Ellison AM, Bank MS, Clinton BD et al (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486. https://doi.org/10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2
Flinn KM, Marks PL (2007) Agricultural legacies in forest environments: tree communities, soil properties, and light availability. Ecol Appl 17:452–463. https://doi.org/10.1890/05-1963
Frelich LE, Calcote RR, Davis MB, Pastor J (1993) Patch formation and maintenance in an old-growth hemlock-hardwood forest. Ecology 74:513–527. https://doi.org/10.2307/1939312
Galante TE, Horton TR, Swaney DP (2011) 95% of basidiospores fall within 1 m of the cap: a field-and modeling-based study. Mycologia 103:1175–1183. https://doi.org/10.3852/10-388
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardes M, Bruns TD (1996) ITS-RFLP matching for identification of fungi. In: Clapp JP (ed) Species Diagnostics Protocols: PCR and Other Nucleic acid Methods. Humana Press, Totowa, pp 177–186
Glassman SI, Peay KG, Talbot JM et al (2015) A continental view of pine-associated ectomycorrhizal fungal spore banks: a quiescent functional guild with a strong biogeographic pattern. New Phytol 205:1619–1631. https://doi.org/10.1111/nph.13240
Godman RM, Lancaster K (1990) Tsuga canadensis (L.) Carr. In: Burns, R. M. and Honkala, B. H., tech. coords. Silvics of North America: Conifers. Washington, DC: U.S. Department of Agriculture, Forest Service, pp 604–612
Gottshall CB, Cooper M, Emery SM (2017) Activity, diversity and function of arbuscular mycorrhizae vary with changes in agricultural management intensity. Agric Ecosyst Environ 241:142–149. https://doi.org/10.1016/j.agee.2017.03.011
Grove S, Saarman NP, Gilbert GS et al (2019) Ectomycorrhizas and tree seedling establishment are strongly influenced by forest edge proximity but not soil inoculum. Ecol Appl 29:e01867. https://doi.org/10.1002/eap.1867
Hoeksema JD, Chaudhary VB, Gehring CA et al (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Holste EK, Kobe RK, Gehring CA (2017) Plant species differ in early seedling growth and tissue nutrient responses to arbuscular and ectomycorrhizal fungi. Mycorrhiza 27:211–223. https://doi.org/10.1007/s00572-016-0744-x
Horton TR (2002) Molecular approaches to ectomycorrhizal diversity studies: variation in ITS at a local scale. Plant Soil 244:29–39. https://doi.org/10.1023/A:1020268020563
Horton TR, Bruns TD, Parker VT (1999) Ectomycorrhizal fungi associated with Arctostaphylos contribute to Pseudotsuga menziesii establishment. Can J Bot 77:93–102. https://doi.org/10.1139/b98-208
Hummer KE (2000) History of the origin and dispersal of white pine blister rust. HortTechnology 10:515–517
Izzo A, Canright M, Bruns TD (2006) The effects of heat treatments on ectomycorrhizal resistant propagules and their ability to colonize bioassay seedlings. Mycol Res 110:196–202. https://doi.org/10.1016/j.mycres.2005.08.010
Jo I, Fei S, Oswalt CM et al (2019) Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci Adv 5:eaav6358. https://doi.org/10.1126/sciadv.aav6358
Jonsson LM, Nilsson M-C, Wardle DA, Zackrisson O (2001) Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93:353–364. https://doi.org/10.1034/j.1600-0706.2001.930301.x
Kårén O, Högberg N, Dahlberg A et al (1997) Inter- and intraspecific variation in the ITS region of rDNA of ectomycorrhizal fungi in Fennoscandia as detected by endonuclease analysis. New Phytol 136:313–325. https://doi.org/10.1046/j.1469-8137.1997.00742.x
Kennedy PG, Smith DP, Horton TR, Molina RJ (2012) Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forests by promoting mycorrhizal fungal diversity and host connectivity. Am J Bot 99:1691–1701. https://doi.org/10.3732/ajb.1200277
Lilleskov EA, Bruns TD, Horton TR et al (2004) Detection of forest stand-level spatial structure in ectomycorrhizal fungal communities. FEMS Microbiol Ecol 49:319–332. https://doi.org/10.1016/j.femsec.2004.04.004
Matlack GR (1989) Secondary dispersal of seed across snow in Betula lenta, a gap-colonizing tree species. Jecol 77:853–869. https://doi.org/10.2307/2260990
Matsuda Y, Takano Y, Shimada H et al (2013) Distribution of ectomycorrhizal fungi in a Chamaecyparis obtusa stand at different distances from a mature Quercus serrata tree. Mycoscience 54:260–264. https://doi.org/10.1016/j.myc.2012.09.019
McEuen AB, Curran LM (2004) Seed dispersal and recruitment limitation across spatial scales in temperate forest fragments. Ecology 85:507–518. https://doi.org/10.1890/03-4006
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574. https://doi.org/10.1890/05-1173
Montesinos-Navarro A, Valiente-Banuet A, Verdú M (2019) Plant facilitation through mycorrhizal symbiosis is stronger between distantly related plant species. New Phytol 224:928–935. https://doi.org/10.1111/nph.16051
Mueller-Dombois D, Ellenberg H (1974) Aims and Methods of Vegetation Ecology. Wiley and Sons, New York
Nara K (2006a) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178. https://doi.org/10.1111/j.1469-8137.2005.01545.x
Nara K (2006b) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198. https://doi.org/10.1111/j.1469-8137.2006.01744.x
Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90:2352–2359. https://doi.org/10.1890/08-2139.1
Peay KG, Garbelotto M, Bruns TD (2010) Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology 91:3631–3640
Peay KG, Schubert MG, Nguyen NH, Bruns TD (2012) Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol Ecol 21:4122–4136. https://doi.org/10.1111/j.1365-294X.2012.05666.x
Perry DA, Molina R, Amaranthus MP (1987) Mycorrhizae, mycorrhizospheres, and reforestation: current knowledge and research needs. Can J Res 17:929–940. https://doi.org/10.1139/x87-145
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51. https://doi.org/10.1111/nph.12221
Policelli N, Horton TR, Hudon AT et al (2020) Back to roots: the role of ectomycorrhizal fungi in boreal and temperate forest restoration. Front for Glob Change 3:1–15
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ribbens E, Silander JA, Pacala SW (1994) Seedling recruitment in forests: calibrating models to predict patterns of tree seedling dispersion. Ecology 75:1794–1806. https://doi.org/10.2307/1939638
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. https://doi.org/10.1111/j.1461-0248.2004.00620.x
Rogers RS (1978) Forests dominated by hemlock (Tsuga canadensis): distribution as related to site and postsettlement history. Can J Bot 56:843–854. https://doi.org/10.1139/b78-096
Schoch CL, Seifert KA, Huhndorf S et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci 109:6241–6246. https://doi.org/10.1073/pnas.1117018109
Service USF, Bonner FT (2008) The Woody Plant Seed Manual. U.S. Department of Agriculture, Forest Service
Smith SE, Read DJ (2010) Mycorrhizal Symbiosis. Academic Press, Cambridge
Snyder CD, Young JA, Lemarié DP, Smith DR (2002) Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Can J Fish Aquat Sci 59:262–275. https://doi.org/10.1139/f02-003
Southgate EWBR, Thompson JE (2014) Secondary forest succession in a post-agricultural landscape in the Hudson Valley, New York. Northeast Nat 21:35–50. https://doi.org/10.1656/045.021.0120
Sýkorová Z, Rydlová J, Slavíková R et al (2016) Forest reclamation of fly ash deposit: a field study on appraisal of mycorrhizal inoculation. Restor Ecol 24:184–193. https://doi.org/10.1111/rec.12301
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850. https://doi.org/10.1046/j.1365-294x.1999.00773.x
Thiet RK, Boerner REJ (2007) Spatial patterns of ectomycorrhizal fungal inoculum in arbuscular mycorrhizal barrens communities: implications for controlling invasion by Pinus virginiana. Mycorrhiza 17:507–517. https://doi.org/10.1007/s00572-007-0123-8
Thompson JR, Carpenter DN, Cogbill CV, Foster DR (2013) Four centuries of change in northeastern United States forests. PLoS One 8:e72540. https://doi.org/10.1371/journal.pone.0072540
Trappe JM (1962) Cenococcum graniforme-its distribution, ecology, mycorrhiza formation, and inherent variation. Doctoral Dissertation, University of Washington
van der Heijden MGA, Klironomos JN, Ursic M et al (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Vellend M, Verheyen K, Flinn KM et al (2007) Homogenization of forest plant communities and weakening of species–environment relationships via agricultural land use. J Ecol 95:565–573. https://doi.org/10.1111/j.1365-2745.2007.01233.x
Weckel M, Tirpak JM, Nagy C, Christie R (2006) Structural and compositional change in an old-growth eastern hemlock Tsuga canadensis forest, 1965–2004. For Ecol Manag 231:114–118. https://doi.org/10.1016/j.foreco.2006.05.022
White BT, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: White TJ, Bruns TD, Lee SB, Taylor JW (eds) PCR - protocols and applications - a laboratory manual. Academic Press, Cambridge, pp 315–322
Whitney GG (1990) The history and status of the hemlock-hardwood forests of the Allegheny Plateau. Jecol 78:443–458. https://doi.org/10.2307/2261123
Yamasaki M, DeGraaf RM, Lanier JW (2000) Wildlife habitat associations in eastern hemlock - birds, smaller mammals, and forest carnivores. Proceedings: Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America, pp 135–143
Acknowledgements
This research would not have been possible without funding and logistical support from the Mianus River Gorge and their staff. We thank Stephen Stehman and Jonathan Cohen for their valuable advice regarding statistical analysis and experimental design. Shawnee Kasanke provided helpful suggestions to improve the manuscript before submission. We also thank Chuck Schirmer for his assistance with soil analyses as well as Summer Blitz, Alice Roosevelt, and Ian Jablonski who helped with laboratory work.
Funding
Funding was provided by a Mianus River Gorge Research Assistant Program Fellowship, Edna Bailey Sussman Foundation internship, SUNY ESF Graduate Student Association grant, and a Lowe-Wilcox scholarship.
Author information
Authors and Affiliations
Contributions
AC and TH conceived and designed the study and AC performed experiments, laboratory work, as well as data analysis. AC and TH wrote and edited all versions of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cortese, A.M., Horton, T.R. Islands in the shade: scattered ectomycorrhizal trees influence soil inoculum and heterospecific seedling response in a northeastern secondary forest. Mycorrhiza 33, 33–44 (2023). https://doi.org/10.1007/s00572-023-01104-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-023-01104-w