Abstract
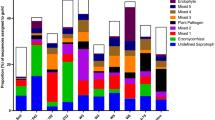
American chestnut (Castanea dentata) was once a dominant overstory tree in eastern USA but was decimated by chestnut blight (Cryphonectria parasitica). Blight-resistant chestnut is being developed as part of a concerted restoration effort to bring this heritage tree back. Here, we evaluate the potential of field soils in the northern portion of the chestnut's former range to provide ectomycorrhizal (EM) fungus inoculum for American chestnut. In our first study, chestnut seedlings were grown in a growth chamber using soil collected from three sites dominated by red oak (Quercus rubra) as inoculum and harvested after 5 months. Of the 14 EM fungi recovered on these seedlings, four species dominated in soils from all three sites: Laccaria laccata, a Tuber sp., Cenococcum geophilum, and a thelephoroid type. Seedlings grown in the nonsterilized soils were smaller than those growing in sterilized soils. In the second study, chestnut seedlings were grown from seed planted directly into soils at the same three sites. Seedlings with intermingling roots of established trees of various species were harvested after 5 months. Seventy-one EM fungi were found on the root tips of the hosts, with 38 occurring on chestnut seedlings. Multiple versus single host EM fungi were significantly more abundant and frequently encountered. The fungi observed dominating on seedlings in the laboratory bioassay were not frequently encountered in the field bioassay, suggesting that they may not have been active in mycelial networks in the field setting but were in the soils as resistant propagules that became active in the bioassay. These results show that soil from red oak stands can be used to inoculate American chestnut with locally adapted ectomycorrhizal fungi prior to outplanting, a relatively cost effective approach for restoration efforts.
Similar content being viewed by others
References
Agerer R (1987) Colour atlas of ectomycorrhizae. Einhorn-verlag, Schwäbisch Gmünd
Anagnostakis SL (2001) The effect of multiple importations of pests and pathogens on a native tree. Biol Invasions 3:245–254. doi:10.1023/A:1015205005751
Ashkannejhad S, Horton TR (2006) Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytol 169:345–354. doi:10.1111/j.1469-8137.2005.01593.x
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytol 143:409–418
Blom JM, Vannini A, Vettraino AM, Hale MD, Goldbold DL (2009) Ectomycorrhizal community structure in a healthy and a Phytophthora-infected chestnut (Castanea sativa Mill.) stand in central Italy. Mycorrhiza 20:25–38
Booth MG (2004) Mycorrhizal networks mediate overstorey–understorey competition in a temperate forest. Ecol Lett 7:538–546. doi:10.1111/j.1461-0248.2004.00605.x
Braun EL (1950) Deciduous forests of eastern North America. Blakiston Co., Philadelphia
Brewer LG (1995) Ecology of survival and recovery from blight in American chestnut trees (Castanea-dentata (Marsh) Borkh) in Michigan. Bull Torrey Bot Club 122:40–57. doi:10.2307/2996402
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with Mycorrhizas in Forestry and Agriculture. ACIAR Monograph, Canberra
Bruns TD, Peay KG, Boynton PJ, Grubisha LC, Hynson NA, Nguyen NH, Rosenstock NP (2009) Inoculum potential of Rhizopogon spores increases with time over the first 4 yr of a 99-yr spore burial experiment. New Phytol 181:463–470. doi:10.1111/j.1469-8137.2008.02652.x
Castellano MA (1996) Outplanting performance of mycorrhizal inoculated seedlings. In: Mukerji KG (ed) Concepts in mycorrhizal research. Kluwer Academic, Dordrecht, pp 223–301
Castellano MA, Trappe JM (1985) Ectomycorrhizal formation and plantation performance of Douglas-fir nursery stock inoculated with Rhizopogon spores. Can J For Res 15:613–617
Cline ET, Ammirati JF, Edmonds RL (2005) Does proximity to mature trees influence ectomycorrhizal fungus communities of Douglas-fir seedlings? New Phytol 166:993–1009
Colpaert JV, Van Assche JA, Luijtens K (1992) The growth of the extramatrical mycelium of ectomyorrhizal fungi and the growth response of Pinus sylvestris L. New Phytol 120:127–135
Dickie IA, Koide RT, Steiner KC (2002) Influences of established trees on mycorrhizas, nutrition, and growth of Quercus rubra seedlings. Ecol Monogr 72:505–521. doi:10.2307/3100054
Dickie IA, Montgomery RA, Reich PB, Schnitzer SA (2007) Physiological and phenological responses of oak seedlings to oak forest soil in the absence of trees. Tree Physiol 27:133–140
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for Basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
Gardes M, Bruns TD (1996) ITS-RFLP matching for the identification of fungi. In: Clapp JP (ed) Methods in molecular biology, vol. 50: species diagnostics protocols: PCR and other nucleic acid methods. Humana, Totowa, pp 177–186
Griffin GJ (1989) Incidence of chestnut blight and survival of American chestnut in forest clear-cut and neighboring understory sites. Plant Dis 73:123–127. doi:10.1094/pd-73-0123
Griffin GJ (1992) American chestnut survival in understory mesic sites following the chestnut blight pandemic. Can J Bot 70:1950–1956
Hebard FV (2006) The backcross breeding program of the American chestnut foundation. In: Steiner KC, Carlson JE (eds) Restoration of American chestnut to forest lands—proceedings of a conference and workshop. May 4–6, 2004, The North Carolina Arboretum, Natural Resources Report NPS/NCR/CUE/NRR - 2006/001. National Park Service, Washington
Horton TR (2002) Molecular approaches to ectomycorrhizal diversity studies: variation in ITS at a local scale. Plant Soil 244:29–39
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10:1855–1871
Horton TR, van der Heijden MGA (2008) The role of symbiosis in seedling establishment and survival. In: Leck M, Parker VT, Simpson RL (eds) Seedling ecology and evolution. Cambridge University Press, Cambridge, pp 150–171
Horton TR, Bruns TD, Parker VT (1999) Ectomycorrhizal fungi associated with Arctostaphylos contribute to Pseudotsuga menziesii establishment. Can J Bot 77:93–102
Horton TR, Molina R, Hood K (2005) Douglas-fir ectomycorrhizae in 40- and 400-year-old stands: mycobiont availability to late successional western hemlock. Mycorrhiza 15:393–403. doi:10.1007/s00572-004-0339-9
Jacobs DF (2007) Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biol Conserv 137:497–506. doi:10.1016/j.biocon.2007.03.013
Jacobs DF, Dalgleish HJ, Nelson CD (2013) A conceptual framework for restoration of threatened plants: the effective model of American chestnut (Castanea dentata) reintroduction. New Phytol 197:378–393
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585
Kåren O, Hogberg N, Dahlberg A, Jonsson L, Nylund JE (1997) Inter- and intraspecific variation in the ITS region of rDNA of ectomycorrhizal fungi in Fennoscandia as detected by endonuclease analysis. New Phytol 136:313–325. doi:10.1046/j.1469-8137.1997.00742.x
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kropp BR, Langlois CG (1990) Ectomycorrhizae in reforestation. Can J For Res 20:438–451. doi:10.1139/x90-061
Lesica P, Allendorf FW (1999) Ecological genetics and the restoration of plant communities: mix or match? Restor Ecol 7:42–50. doi:10.1046/j.1526-100X.1999.07105.x
Merkle SA, Andrade GM, Nairn CJ, Powell WA, Maynard CA (2007) Restoration of threatened species: a noble cause for transgenic trees. Tree Genet Genomes 3:111–118. doi:10.1007/s11295-006-0050-4
Michelangeli FA, Penneys DS, Giza J, Soltis D, Hils MH, Skean JD (2004) A preliminary phylogeny of the tribe Miconieae (Melastomataceae) based on nrITS sequence data and its implications on inflorescence position. Taxon 53:279–290. doi:10.2307/4135608
Molina R, Trappe JM (1984) Mycorrhiza management in bareroot nurseries. In: Duryea ML, Landis TD (eds) Forest nursery manual: production of bareroot seedlings. Martinus Nijhoff/Dr W. Junk Publishers. The Hague/Boston/Lancaster, for Forest Research Laboratory, Oregon State University, Corvallis, pp 211-223
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbioses: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman and Hall, New York, pp 357–423
Morgan P (1984) Pacific Northwest forest nursery mycorrhizae research: boon or boondoggle? In: Molina R (ed) Proceedings of the sixth North American conference on mycorrhizae, Bend, Oregon. Forest Research Laboratory, Corvallis, pp 73–74
Nara K (2006a) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178. doi:10.1111/j.1469-8137.2005.01545.x
Nara K (2006b) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198. doi:10.1111/j.8137.2006.01744.x
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707. doi:10.1890/03-0373
Newman EI (1988) Mycorrhizal links between plants-their functioning and ecological significance. Adv Ecol Res 18:243–270. doi:10.1016/s0065-2504(08)60182-8
Onguene NA, Kuyper TW (2002) Importance of the ectomycorrhizal network for seedling survival and ectomycorrhiza formation in rain forests of South Cameroon. Mycorrhiza 12:13–17. doi:10.1007/s00572-001-0140-y
Paillet FL (1984) Growth-form and ecology of American chestnut sprout clones in northeastern Massachusetts. Bull Torrey Bot Club 111:316–328. doi:10.2307/2995913
Paillet FL (1993) Growth form and life-histories of American chestnut and Allegheny and Ozark chinquapin at various North-American sites. Bull Torrey Bot Club 120:257–268. doi:10.2307/2996990
Palmer J, Lindner D, Volk T (2008) Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in western Wisconsin. Mycorrhiza 19:27–36. doi:10.1007/s00572-008-0200-7
Perry DA, Molina R, Amaranthus MP (1987) Mycorrhizae, mycorrhizospheres, and reforestation—current knowledge and research needs. Can J For Res 17:929–940. doi:10.1139/x87-145
Polin LD, Liang HY, Rothrock RE, Nishii M, Diehl DL, Newhouse AE, Nairn CJ, Powell WA, Maynard CA (2006) Agrobacterium-mediated transformation of American chestnut (Castanea dentata (Marsh.) Borkh.) somatic embryos. Plant Cell Tiss Org 84:69–78. doi:10.1007/s11240-005-9002-1
Quoreshi AM, Timmer VR (2000) Early outplanting performance of nutrient-loaded containerized black spruce seedlings inoculated with Laccaria bicolor: a bioassay study. Can J For Res 30:744–752. doi:10.1139/cjfr-30-5-744
Rappalie DF (1981) Soil survey of Oswego County, New York. U.S. Department of Agriculture, Washington
Roane M, Griffin GJ, Elkins JR (1986) Chestnut blight, other endothia diseases, and the genus Endothia. American Phytopathological Society, St. Paul
Rothrock RE, Polin-McGuigan LD, Newhouse AE, Powell WA, Maynard CA (2007) Plate flooding as an alternative Agrobacterium-mediated transformation method for American chestnut somatic embryos. Plant Cell Tiss Org 88:93–99. doi:10.1007/s11240-006-9170-7
SAS Institute Inc (2002) SAS version 9.0. SAS Institute, Inc, Cary
Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A (2006) The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol Lett 9:501–515. doi:10.1111/j.1461-0248.2006.00910.x
Sinclair WA, Sylvia DM, Larsen AO (1982) Disease suppression and growth promotion in Douglas-fir seedlings by the ectomycorrhizal fungus Laccaria laccata. Forest Sci 28:191–201
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109. doi:10.1007/bf00037152
Tarbell TJ, Koske RE (2007) Evaluation of commercial arbuscular mycorrhizal inocula in a sand/peat medium. Mycorrhiza 18:51–56. doi:10.1007/s00572-007-0152-3
Taylor AFS (2002) Fungal diversity in ectomycorrhizal communities: sampling effort and species detection. Plant Soil 244:19–28. doi:10.1023/a:1020279815472
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850. doi:10.1046/j.1365-294x.1999.00773.x
Trappe JM (1977) Selection of fungi for ectomycorrhizal inoculation in nurseries. Annu Rev Phytopathol 15:203–222
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97:1139–1150
Villeneuve N, Le Tacon F, Bouchard D (1991) Survival of inoculated Laccaria bicolor in competition with native ectomycorrhizal fungi and effects on the growth of outplanted Douglas-fir seedlings. Plant Soil 135:95–107. doi:10.1007/bf00014782
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application. Academic, New York, pp 315–322
Acknowledgments
This project partly fulfilled an MS degree at the State University of New York College of Environmental Science and Forestry (K.M.D.). We thank the New York chapter of the American Chestnut Foundation for their help in collecting seeds and general interest in the project. Al Nichols, Stan Warsig, and Arlene Warsig were particularly helpful. Financial support was provided by National Research Initiative award no. 99-35107-7843 from the USDA Cooperative State Research Education and Extension Service (T.R.H.), National Science Foundation award DEB-0614381 (T.R.H.), and the Sussman Foundation (K.M.D.).
Disclaimer
The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Dulmer, K.M., LeDuc, S.D. & Horton, T.R. Ectomycorrhizal inoculum potential of northeastern US forest soils for American chestnut restoration: results from field and laboratory bioassays. Mycorrhiza 24, 65–74 (2014). https://doi.org/10.1007/s00572-013-0514-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-013-0514-y