Abstract
Chronic pancreatitis (CP) is a major risk factor for pancreatic cancer; however, little is known about the pathogenic mechanisms underlying the development of CP. Legumain (Lgmn) has been linked to some chronic inflammatory diseases. The present study investigated the role of legumain in pancreatic fibrogenesis. We induced CP in wild type C57BL6 (WT), Lgmn-deficient (Lgmn−/−), Lgmnflox/flox and Lgmnflox/flox × LysMCre mice by intraperitoneal injection of caerulein for 4 weeks. Pancreata were collected and analyzed by quantitative reverse transcription polymerase chain reaction, Western blotting, and histology. Pancreatic stellate cells and macrophages were isolated and studied using immunofluorescence, gelatin zymography, and enzyme-linked immunosorbent assay. The effects of inhibition of legumain were investigated in vivo by administration of the specific legumain inhibitor, RR-11a. Legumain was found to be upregulated in the serum and pancreatic tissues of mice with caerulein-induced CP. Mice with global and macrophage-specific legumain deficiency exhibited significantly reduced development of pancreatic fibrosis compared with control mice, based on pancreas size, histology, and expression of fibrosis-associated genes. Our results indicate that legumain promotes activation of pancreatic stellate cells and increases synthesis of extracellular matrix proteins via activation of matrix metalloproteinase-2(MMP-2), which hydrolyzes the transforming growth factor-β1 (TGF-β1) precursor to form active TGF-β1. Administration of RR-11a markedly attenuated pancreatic fibrosis in mice with CP. Deficiency or inhibition of legumain significantly reduces the severity of pancreatic fibrosis by suppressing activation of the TGF-β1 precursor. Our results highlight the potential of legumain as a novel therapeutic target for CP.
Key messages
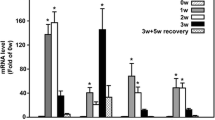
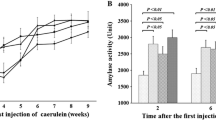
• Legumain expression was markedly upregulated in CP mice.
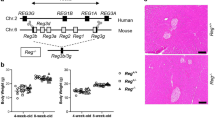
• Deletion of legumain attenuated pancreatic fibrosis in CP mice.
• Legumain promotes fibrosis via MMP-2 activation, which hydrolyzed the TGF-β1 precursor to the active form.
• Legumain is a potential therapeutic target for the management of CP.







Similar content being viewed by others
References
Kloppel G (2007) Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 20(Suppl 1):S113–S131
Hoffmeister A, Mayerle J, Beglinger C et al (2015) English language version of the S3-consensus guidelines on chronic pancreatitis: definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Z Gastroenterol 53:1447–1495
Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, Hewitt E, Watts C, Barrett AJ (1997) Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem 272:8090–8098
Dall E, Brandstetter H (2016) Structure and function of legumain in health and disease. Biochimie 122:126–150
Watts C, Matthews SP, Mazzeo D, Manoury B, Moss CX (2005) Asparaginyl endopeptidase: case history of a class II MHC compartment protease. Immunol Rev 207:218–228
Sepulveda FE, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, Lennon-Duménil AM, Amigorena S, Cabanie L, Manoury B (2009) Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity 31:737–748
Liu Z, Jang SW, Liu X, Cheng D, Peng J, Yepes M, Li XJ, Matthews S, Watts C, Asano M, Hara-Nishimura I, Luo HR, Ye K (2008) Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell 29:665–678
Mattock KL, Gough PJ, Humphries J, Burnand K, Patel L, Suckling KE, Cuello F, Watts C, Gautel M, Avkiran M, Smith A (2010) Legumain and cathepsin-L expression in human unstable carotid plaque. Atherosclerosis 208:83–89
Miller G, Matthews SP, Reinheckel T, Fleming S, Watts C (2011) Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J 25:1606–1617
Chen JM, Fortunato M, Stevens RA, Barrett AJ (2001) Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol Chem 382:777–783
Liu C, Sun C, Huang H, Janda K, Edgington T (2003) Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res 63:2957–2964
Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, Lane Z, Crispin DA, Goodlett DR, Aebersold R, Bronner MP (2007) Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics 6:1331–1342
Edgington-Mitchell LE, Wartmann T, Fleming AK, Gocheva V, van der Linden W, Withana NP, Verdoes M, Aurelio L, Edgington-Mitchell D, Lieu T, Parker BS, Graham B, Reinheckel T, Furness JB, Joyce JA, Storz P, Halangk W, Bogyo M, Bunnett NW (2016) Legumain is activated in macrophages during pancreatitis. Am J Physiol Gastrointest Liver Physiol 311:G548–G560
Bai P, Lyu L, Yu T et al (2019) Macrophage-derived legumain promotes pulmonary hypertension by activating the MMP (matrix metalloproteinase)-2/TGF (transforming growth factor)-beta1 signaling. Arterioscler Thromb Vasc Biol 39:e130–e145
Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, Edderkaoui M, Pandol SJ, Park W, Habtezion A (2016) Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology 151:1206–1217
Witt H, Apte MV, Keim V, Wilson JS (2007) Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 132:1557–1573
Lerch MM, Gorelick FS (2013) Models of acute and chronic pancreatitis. Gastroenterology 144:1180–1193
Xue J, Sharma V, Hsieh MH et al (2015) Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 6:7158
Song L, Chen TY, Zhao XJ, Xu Q, Jiao RQ, Li JM, Kong LD (2019) Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. Br J Pharmacol 176:1619–1634
Loboda A, Sobczak M, Jozkowicz A et al (2016) TGF-beta1/smads and miR-21 in renal fibrosis and inflammation. Mediat Inflamm 2016:8319283
Xu M, Wang G, Zhou H et al (2017) TGF-beta1-miR-200a-PTEN induces epithelial-mesenchymal transition and fibrosis of pancreatic stellate cells. Mol Cell Biochem 431:161–168
Miyamoto T, Nakamura H, Nagashio Y, Asaumi H, Harada M, Otsuki M (2010) Overexpression of Smad6 exacerbates pancreatic fibrosis in murine caerulein-induced chronic pancreatic injuries. Pancreas 39:385–391
Wang D, Xiong M, Chen C, du L, Liu Z, Shi Y, Zhang M, Gong J, Song X, Xiang R, Liu E, Tan X (2018) Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int 94:91–101
Luo YP, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R (2006) Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 116:2132–2141
Choi SJ, Reddy SV, Devlin RD, Menaa C, Chung H, Boyce BF, Roodman GD (1999) Identification of human asparaginyl endopeptidase (legumain) as an inhibitor of osteoclast formation and bone resorption. J Biol Chem 274:27747–27753
Sendler M, Weiss FU, Golchert J et al (2018) Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology 154(704–718):e10
Weber H, Huhns S, Luthen F et al (2005) Calpain activation contributes to oxidative stress-induced pancreatic acinar cell injury. Biochem Pharmacol 70:1241–1252
Lyo V, Cattaruzza F, Kim TN, Walker AW, Paulick M, Cox D, Cloyd J, Buxbaum J, Ostroff J, Bogyo M, Grady EF, Bunnett NW, Kirkwood KS (2012) Active cathepsins B, L, and S in murine and human pancreatitis. Am J Physiol Gastrointest Liver Physiol 303:G894–G903
Ramadani M, Yang Y, Gansauge F, Gansauge S, Beger HG (2001) Overexpression of caspase-1 (interleukin-1beta converting enzyme) in chronic pancreatitis and its participation in apoptosis and proliferation. Pancreas 22:383–387
Solberg R, Smith R, Almlof M et al (2015) Legumain expression, activity and secretion are increased during monocyte-to-macrophage differentiation and inhibited by atorvastatin. Biol Chem 396:71–80
Principe DR, DeCant B, Mascarinas E et al (2016) TGFbeta signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Cancer Res 76:2525–2539
Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D, Alberti S, Chen D, Zuo S, Zhang Q, Yan S, Fei X, Yuan F, Zhou B, Duan S, Yu Y, Lazarus M, Su Y, Breyer RM, Funk CD, Yu Y (2015) EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-beta1 signaling. J Clin Invest 125:1228–1242
Vogelmann R, Ruf D, Wagner M, Adler G, Menke A (2001) Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol 280:G164–G172
Kordes C, Brookmann S, Haussinger D et al (2005) Differential and synergistic effects of platelet-derived growth factor-BB and transforming growth factor-beta1 on activated pancreatic stellate cells. Pancreas 31:156–167
Shek FWT, Benyon RC, Walker FM, McCrudden P, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, Iredale JP (2002) Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160:1787–1798
Robertson IB, Rifkin DB (2013) Unchaining the beast; insights from structural and evolutionary studies on TGFbeta secretion, sequestration, and activation. Cytokine Growth Factor Rev 24:355–372
Wipff PJ, Hinz B (2008) Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol 87:601–615
Stetler-Stevenson WG, Aznavoorian S, Liotta LA (1993) Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9:541–573
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18:816–827
Liu X, Wang Z, Zhang G, Zhu Q, Zeng H, Wang T, Gao F, Qi Z, Zhang J, Wang R (2017) High TRAF6 expression is associated with esophageal carcinoma recurrence and prompts cancer cell invasion. Oncol Res 25:485–493
Acknowledgments
We thank Professor Ankang Lyu (Department of Cardiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for providing Lgmn−/−, Lgmnflox/flox, and LysMCre mice for this study. We would also like to thank Dr. Peiyuan Bai (Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China) for valuable suggestions and technical assistance on our experiments.
Funding
This work was sponsored by Natural Science Foundation of China grants to Gongyong Hu (81670584 and 81970556) and Xingpeng Wang (81570580), and the Shanghai Pujiang Program to Gongyong Hu (18PJD041).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experiments were performed in accordance with the principle for replacement, refinement, and reduction (the 3Rs) and were approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine (SYXK 2013-0050, Shanghai, China.).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, YC., Zhao, Q., He, Y. et al. Legumain promotes fibrogenesis in chronic pancreatitis via activation of transforming growth factor β1. J Mol Med 98, 863–874 (2020). https://doi.org/10.1007/s00109-020-01911-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01911-0