Abstract
Background and Aims
Chronic pancreatitis is associated with recurrent inflammation, pain, fibrosis, and loss of exocrine and endocrine pancreatic function and risk of cancer. We hypothesized that activation of the CCK receptor contributes to pancreatitis and blockade of this pathway would improve chronic pancreatitis.
Methods
Two murine models were used to determine whether CCK receptor blockade with proglumide could prevent and reverse histologic and biochemical features of chronic pancreatitis: the 6-week repetitive chronic cerulein injection model and the modified 75% choline-deficient ethionine (CDE) diet. In the CDE-fed model, half the mice received water supplemented with proglumide, for 18 weeks. After chronic pancreatitis was established in the cerulein model, half the mice were treated with proglumide and half with water. Histology was scored in a blinded fashion for inflammation, fibrosis and acinar ductal metaplasia (ADM) and serum lipase levels were measured. RNA was extracted and examined for differentially expressed fibrosis genes.
Results
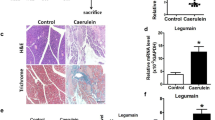
Proglumide therapy decreased pancreatic weight in the CDE diet study and the cerulein-induced chronic pancreatitis model. Fibrosis, inflammation, and ADM scores were significantly reduced in both models. Lipase values improved with proglumide but not in controls in both models. Proglumide decreased pancreas mRNA expression of amylase, collagen-4, and TGFβR2 gene expression by 44, 38, and 25%, respectively, compared to control mice.
Conclusion
New strategies are needed to decreased inflammation and reduce fibrosis in chronic pancreatitis. CCK receptor antagonist therapy may improve chronic pancreatitis by reversing fibrosis and inflammation. The decrease in ADM may reduce the risk of the development of pancreatic cancer.






Similar content being viewed by others
Abbreviations
- ADM:
-
Acinar ductal metaplasia
- CCK:
-
Cholecystokinin
- CDE:
-
Choline-deficient ethionine
- MMP:
-
Matrix metalloproteinase
- PSC:
-
Pancreatic stellate cells
References
Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218–224.
Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261.
Conwell DL, Wu BU. Chronic pancreatitis: making the diagnosis. Clin Gastroenterol Hepatol. 2012;10:1088–1095.
Dhar P, Kalghatgi S, Saraf V. Pancreatic cancer in chronic pancreatitis. Indian J Surg Oncol. 2015;6:57–62.
Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1366–1372.
Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921.
Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–S54.
Trautwein C, Friedman SL, Schuppan D, et al. Hepatic fibrosis: concept to treatment. J Hepatol. 2015;62:S15–S24.
Apte M, Pirola RC, Wilson JS. Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr Opin Gastroenterol. 2015;31:416–423.
Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162.
Lieb JG, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:706–719.
Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528.
Bedossa P. Reversibility of hepatitis B virus cirrhosis after therapy: who and why? Liver Int. 2015;35:78–81.
Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41.
Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39:S60–S63.
Zimmermann A, Gloor B, Kappeler A, et al. Pancreatic stellate cells contribute to regeneration early after acute necrotising pancreatitis in humans. Gut. 2002;51:574–578.
Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193.
Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117.
Yamamoto M, Otani M, Otsuki M. A new model of chronic pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G700–G708.
Tanaka T, Miura Y, Matsugu Y, et al. Pancreatic duct obstruction is an aggravating factor in the canine model of chronic alcoholic pancreatitis. Gastroenterology. 1998;115:1248–1253.
Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082.
Meyerholz DK, Stoltz DA, Pezzulo AA, et al. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389.
Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975;79:465–480.
Ida S, Ohmuraya M, Hirota M, et al. Chronic pancreatitis in mice by treatment with choline-deficient ethionine-supplemented diet. Exp Anim. 2010;59:421–429.
Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007;14:63–67.
Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847.
Singh P, Owlia A, Espeijo R et al. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995;270:8429–8438.
Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914.
Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187.
Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219.
Smith JP, Cooper TK, McGovern CO, et al. Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice. Pancreas. 2014;43:1050–1059.
Nadella S, Burks J, Al-Sabban A, et al. Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor. Am J Physiol Gastrointest Liver Physiol. 2018;315:G699–G712.
Passman AM, Strauss RP, McSpadden SB, et al. A modified choline-deficient, ethionine-supplemented diet reduces morbidity and retains a liver progenitor cell response in mice. Dis Model Mech. 2015;8:1635–1641.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.
Adler G, Gerhards G, Schick J, et al. Effects of in vivo cholinergic stimulation of rat exocrine pancreas. Am J Physiol. 1983;244:G623–G629.
Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88:1192–1204.
Saluja A, Saito I, Saluja M, et al. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol. 1985;249:G702–G710.
Whitcomb DC. Pancreatitis: TIGAR-O version 2 risk/etiology checklist with topic reviews, updates, and use primers. Clin Transl Gastroenterol. 2019;10:e00027.
Ji B, Bi Y, Simeone D, et al. Human pancreatic acinar cells do not respond to cholecystokinin. Pharmacol Toxicol. 2002;91:327–332.
Berna MJ, Jensen RT. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr Top Med Chem. 2007;7:1211–1231.
Chang RS, Lotti VJ, Chen TB, et al. Characterization of the binding of [3H]-(±)-L-364,718: a new potent, nonpeptide cholecystokinin antagonist radioligand selective for peripheral receptors. Mol Pharmacol. 1986;30:212–217.
Boyce M, Warrington S, Black J. Netazepide, a gastrin/CCK2 receptor antagonist, causes dose-dependent, persistent inhibition of the responses to pentagastrin in healthy subjects. Br J Clin Pharmacol. 2013;76:689–698.
Chang KJ. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis and staging of pancreatic tumors. Gastrointest Endosc Clin N Am. 1995;5:723–734.
Pauletzki JG, Xu QW, Shaffer EA. Inhibition of gallbladder emptying decreases cholesterol saturation in bile in the Richardson ground squirrel. Hepatology. 1995;22:325–331.
Miederer SE, Lindstaedt H, Kutz K, et al. Efficient treatment of gastric ulcer with proglumide (Milid) in outpatients (double blind trial). Acta Hepatogastroenterol (Stuttg). 1979;26:314–318.
Brzozowski T, Konturek PC, Konturek SJ, et al. Acceleration of ulcer healing by cholecystokinin (CCK): role of CCK-A receptors, somatostatin, nitric oxide and sensory nerves. Regul Pept. 1999;82:19–33.
Shiratori K, Takeuchi T, Satake K, et al. Clinical evaluation of oral administration of a cholecystokinin-A receptor antagonist (loxiglumide) to patients with acute, painful attacks of chronic pancreatitis: a multicenter dose-response study in Japan. Pancreas. 2002;25:e1–e5.
Smith JP, Liu G, Soundararajan V, et al. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am J Physiol. 1994;266:R277–R283.
Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91–G101.
Garces MC, Gomez-Cerezo J, Alba D, et al. Relationship of basal and postprandial intraduodenal bile acid concentrations and plasma cholecystokinin levels with abdominal pain in patients with chronic pancreatitis. Pancreas. 1998;17:397–401.
Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095.
Das SK, Varadhan S, Dhanya L, et al. Diagnostic efficiency of amylase and type IV collagen in predicting chronic pancreatitis. Indian J Clin Biochem. 2009;24:60–64.
Gress TM, Menke A, Bachem M, et al. Role of extracellular matrix in pancreatic diseases. Digestion. 1998;59:625–637.
Nagashio Y, Ueno H, Imamura M, et al. Inhibition of transforming growth factor beta decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1610–1618.
Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17:594–604.
Storz P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2017;14:296–304.
Acknowledgments
We appreciate the technical support from the staff in the Lombardi Core Histology laboratory. We also appreciate the staff in the animal care facility.
Funding
This work was funded by a grant from the National Pancreas Foundation to SN and VC. Part of the work was also funded by an American Gastroenterology Association Elsevier Pilot Research Award to JPS. Postdoctoral support was provided by a NIH training grant to Sandeep Nadella TL1TR001431. These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology and Tissue Shared resource and in the Preclinical Imaging Research Laboratory which is supported in part by NIH/NCI grant P30-CA051008.
Author information
Authors and Affiliations
Contributions
SN, VC, and JPS contributed to conception and design; SN, VC, HC, BK, RDT, and JPS contributed to acquisition of data; analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis) were performed by SN, VC, HC, and JPS; all authors contributed equally to writing, review, and/or revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nadella, S., Ciofoaia, V., Cao, H. et al. Cholecystokinin Receptor Antagonist Therapy Decreases Inflammation and Fibrosis in Chronic Pancreatitis. Dig Dis Sci 65, 1376–1384 (2020). https://doi.org/10.1007/s10620-019-05863-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05863-5