Abstract
Key Message
Beech growth acclimated better during severe drought and recovered faster than spruce after drought ended. This was associated with a shift in performance along relative tree size towards small trees.
Abstract
The effects of several consecutive drought years and the recovery reaction of mature trees in particular after a long-term drought have been poorly studied so far. In this study, we demonstrate the growth reactions of mature trees during and after a five-year treatment of extended summer droughts, followed by controlled irrigation in a very productive mixed forest stand. We exposed 70-year-old Norway spruce (Picea abies [L.] Karst) and 90-year-old European beech (Fagus sylvatica [L.]) trees to reduced precipitation using automatic throughfall exclusion (TE) roofs during the growing seasons from 2014 to 2018, irrigated the trees in early summer 2019 and removed the roofs thereafter. From 2009 to 2022, we monitored annual tree growth and precipitation on 6 plots with throughfall exclusion and on 6 plots with ambient Control conditions (CO) of the KROOF canopy experiment. Norway spruce lost significant growth during drought, with some trees dying and others remaining at a low growth level without significant recovery from the effects of drought stress. European beech also significantly reduced growth at the beginning of the drought but emerged stronger in growth from the drought than the Control group. Spruce and beech showed a non-significant trend of increased inter-specific growth compared to intra-specific growth during drought. We found that spruce benefitted more from mixture than beech in the recovery phase after drought than during the drought phase itself. Most importantly, we observed a shift in growth performance along the relative tree size towards smaller trees in the TE plots for both species. This change in the relationship between diameter increment and tree size during and after drought is a major finding of our study and suggests a possible response mechanism to prolonged drought. This key observation requires further investigation and should be considered in future forest management strategies under changing climatic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change is affecting forests worldwide through altered water and carbon cycles (Breshears et al. 2005; Ciais et al. 2005; Zhao and Running 2010) and through more frequent and intensified drought years, as well as extended arid periods (Allen et al. 2010; IPCC 2014). Drought can disrupt important physiological processes in trees such as water transport and carbon allocation (Adams et al. 2017; Choat et al. 2012, 2018; Hesse et al. 2019), cause various stress symptoms and negatively affect plant growth (Anderegg et al. 2012; Ciais et al. 2005; Gupta et al. 2020; Rais et al. 2021; Walthert et al. 2021). Many studies show that single dry years can reduce growth of Norway spruce (Picea abies [L.] Karst), European beech (Fagus sylvatica [L.]), and other tree species by up to 50% (Bosela et al. 2021; Leuzinger et al. 2005; Lloret et al. 2011; Zang et al. 2012). Recent large-scale studies with large data sets have highlighted even more clearly the effects of climatic variables on spruce and beech growth, with certain variables identified as particularly influential for spruce (Hlásny et al. 2017). A study conducted in the Czech Republic found that severe drought in 2017 and 2018 resulted in a significant decrease in annual radial stem growth of spruce compared to measurements in the wetter year 2016 of 78% and 61%, respectively (Krejza et al. 2021). Rohner et al. (2021) reported about growth reductions for beech trees of up to 100% during the severe spring–summer drought in 2018. Climate change, particularly extended and intensified droughts, causes severe abiotic stress and increases the risk of biotic stress to trees (Allen and Breshears 1998; Bauhus et al. 2017; Dale et al. 2001; Lindner et al. 2010) which may result in increased tree mortality or large-scale disturbance of forests (Allen et al. 2010, 2015; Anderegg et al. 2012; Choat et al. 2018; Hartmann et al. 2018; McDowell et al. 2008; Pretzsch et al. 2020a; Schuldt et al. 2020; Seidl et al. 2017). The partitioning of the stand-level growth results from the partitioning of resources among the individual trees in a stand. To date, there has been little analysis of interindividual growth partitioning and, in particular, the effects of long-term and repeated droughts on the contribution of different population fractions within the stand structure to total stand growth (Pretzsch et al. 2018b; Trouvé et al. 2014). In the long term, the functionality and further persistence of forest stands will be determined by their ability to resist such disturbances or to recover after non-lethal disturbances (Ibáñez et al. 2019). Especially the recovery of mature trees from stress, which is fundamental to understanding the stress resilience of forests, has been far too little addressed (Ruehr et al. 2019).
In recent years, knowledge of the growth effects of mixing complementary tree species has increased (Bosela et al. 2015; del Río et al. 2014a; Pretzsch et al. 2015b; Pretzsch et al. 2017). Pretzsch and Zenner (2017) gave a broad review of the most important mixing effects, illustrated the role of models in the research on mixed-species stands, and pointed out remaining knowledge gaps. Many studies have specifically investigated tree responses to drought stress, showing differences between monocultures and mixed stands (Bréda et al. 2006; Ding et al. 2017; Grams et al. 2021; Kolář et al. 2017; Pretzsch et al. 2014). In many cases, an increase in growth has been confirmed when trees grow in mixed stands (Ammer 2019; Pretzsch et al. 2013, 2020a). The impact of mixture on forest stability and productivity was recently highlighted by del Río et al. (2022), who showed that adding just one more tree species to monocultures increased forest growth productivity and stability (overyielding: + 6%; temporal stability: + 12%). Pardos et al. (2021) found in a large-scale study on 13 trials across nine European countries that mixed forests, especially those combining conifer and deciduous species, have higher resilience and resistance to drought events than monospecific stands. However, this advantage of mixtures could not be generalized. Indeed, these benefits of species mixing can vary according to stand density (Pretzsch and del Río 2020), site conditions (Lévesque et al. 2016; Steckel et al. 2020b), tree species combination (Grossiord et al. 2014; Grossiord 2018; Steckel et al. 2020a), and mix proportion, among other factors (Pretzsch et al. 2020b). When the stress factor is reduced, the advantages can reverse to a disadvantage (Pretzsch et al. 2015a, 2017). Therefore, the influence of species mixing, respectively, intra- and interspecific competition has not been clarified.
Mixed spruce-beech forests cover large areas in Central Europe and are one of the preferred mixtures in actual silviculture (Grams et al. 2021). Both species, Norway spruce and European beech, exhibit species-specific differences in growth, stress reactions, and recovery after stress (Bréda et al. 2006; Bréda et al. 2006; Zang et al. 2012) and promote stand stability under poor conditions (Pretzsch et al. 2010). Reasons for such stabilization might be that Norway spruce and European beech differ in terms of their hydrological anatomy and physiology (Ellenberg and Leuschner 2010). Spruce with tracheids and few stem parenchyma cells is considered to be a very drought-sensitive tree species (Lévesque et al. 2013) with a more isohydric strategy, while beech with xylem vascular elements and higher proportions of xylem parenchyma has an anisohydric strategy (Hartmann 2011; Krupkova et al. 2019; McDowell et al. 2008; Pretzsch et al. 2013). From these two strategies, isohydric plants are often assumed to be more affected by carbon starvation under extended drought (Kannenberg and Phillips 2020). Conversely, anisohydric plants are assumed to be more affected by hydraulic failure and thus water stress, especially during short periods of extreme drought (Grams et al. 2021).
Further complementary factors between spruce and beech are obvious. Spruce is an evergreen gymnosperm with needles and a mostly shallow-rooting system, whereas beech is a deciduous angiosperm with foliage and a heart-root system with coarse roots spreading horizontally and vertically (Grams et al. 2021). The maximum vertical fine root distribution for beech is below that of spruce (Ellenberg and Leuschner 2010). The different strategic approaches to hydraulic function and carbon assimilation, together with complementarity lead to a spatial and temporal offset in resource demands and thus may be checked if they can be considered as an explanation of different response patterns to changing environmental conditions (del Río et al. 2014b).
Although growth partitioning in light-limited temperate forests has been well studied, there is a lack of research on growth partitioning under water-limited conditions, with many studies on drought stress reaction focusing on the growth of dominant trees assuming that these trees reflect the response patterns of the entire stand. Pretzsch et al. (2018b) referred to studies that have investigated the effect of drought stress at different levels of organization (dominant trees vs. whole stand) and suggest that trees with different relative tree sizes respond differently to drought, to not neglect that dominant trees may continue to benefit from their achieved tree size (correlating with their social position), or, as recent results suggest, may show a particular vulnerability to drought (Bennett et al. 2015; Lebourgeois et al. 2014; McGregor et al. 2021; Stovall et al. 2019). For example, Ding et al. (2017) and Zang et al. (2012) report that growth reaction to drought varies as a function of tree size. Accordingly, a distinction can be made between completely symmetric, over size-symmetric to strongly size-asymmetric inter-individual partitioning patterns (Aldea et al. 2021; Pretzsch and Biber 2010). Relative reductions in growth are often more severe on sites previously well supplied with water than on sites that are intrinsically dry (Rukh et al. 2020; Stovall et al. 2019), but may be mitigated by factors such as stand density and tree species mixture (Aussenac et al. 2019; Pretzsch et al. 2015a, 2017; Pretzsch and del Río, 2020). Smaller trees in the stand could have a stabilizing function under dry conditions by partially compensating the losses of their dominant neighbors (Pretzsch and Biber 2010; Wichmann 2001). Studies showed that the correlation between tree size and growth is strong in wet years and weak in dry years. Findings at wet sites compared to dry sites show an analogous pattern. Thus, variation occurs at the temporal scale as well as at the spatial scale (Pretzsch et al. 2018b). Latte et al. (2016) have shown that in addition to such effects of drought on growth distribution in whole stands, there are also changes in growth distribution within trees (along the stem to the crown).
Controlled experiments in greenhouses or phytotrons can only provide limited answers to the mentioned knowledge gaps. To gain new insights, theories need to be tested in natural and mature stands (Grams et al. 2021; Hartmann et al. 2018). So far, field manipulation studies on an ecosystem scale are still very rare but would contribute substantially to the understanding of tree reactions in complex environmental networks (Englund and Cooper 2003; Pretzsch et al. 2018a). Field manipulation studies that ensure both a prolonged drought and recovery through irrigation are even more rare (Gao et al. 2021). First insights into the growth losses in connection with drought stress and possible acclimation and recovery reactions are provided by the KROOF canopy experiment setup in the Kranzberg Forest near Freising (Bavaria) (Grams et al. 2021; Pretzsch et al. 2014, 2020a). Because the experiment in Kranzberg is unique in its design so far, this study is one of the first to present possible recovery reactions of mature spruce and beech trees after five years of drought stress under field conditions and enables to analyze the growth reactions of forests under extreme and extended drought. In this study, we investigated the influence of an extended five-year experimentally induced drought on the growth of mature trees in a stand of Norway spruce and European beech under intra- and inter-specific competition. We studied the possible recovery of growth following irrigation after a long-term drought-period and analyzed whether the relative tree size of individual trees had an effect on growth during drought and on recovery after irrigation. For this study, we concentrated on the following questions and null hypotheses:
-
Q1: Do Norway spruce and European beech recover in their growth rate after extended (5-year-long) experimentally induced drought and subsequent irrigation?
-
H01: Both, Norway spruce and European beech show full growth recovery to the unstressed growth upon irrigation after an extended drought period. Growth recovery is similar for Norway spruce and European beech.
-
Q2: What influence does intra- or inter-specific competition have on the recovery reaction?
-
H02: Growth recovery is independent of intra- and inter-specific environments. Intra-specific responses do not differ from inter-specific responses and all trees in a stand react similarly.
-
Q3: What are the effects of an extended drought and a subsequent irrigation on the growth reaction along the relative size of trees in a stand?
-
H03: Drought stress reaction and growth reaction after drought are independent of relative tree size within the population. All trees irrespective of their relative size in a stand react similarly.
Materials and methods
Site
In our study, we used the KROOF design to show, if spruce and beech growth responds to extended drought, if species mix modifies growth reactions, if both species recover with subsequent irrigation, and if growth collapse and recovery vary along relative tree size. Kranzberg Forest (longitude: 11° 39′ 42″ E, latitude: 48° 25′ 12″ N, altitude 490 m a.s.l) is located in Southern Germany, approximately 35 km Northeast of Munich. At the site, mono-specific and mixed-species stands of Norway spruce and European beech stock on luvisol originating from loess over Tertiary sediments that provide a high nutrient and water supply (Göttlein et al. 2012; Pretzsch et al. 2014). Depending on soil depth, the water holding capacity for plant available water ranges between 17 and 28% of volumetric soil water content, while soil pHH2O varies between 4.1 and 5.1.
Treatments
At the approximately 0.5 ha KROOF experimental site, a total of 12 experimental plots were installed (Fig. 1). Each plot includes a group of 3 to 7 beech and spruce trees at opposing ends of the plot (as of 2014 at the start of the experiment). To examine mixing effects, the plots were designed to create areas of intra-specific competition at each end of each plot and an area for inter-specific competition in the middle. One tree of each species represented the intra-specific competition, and the other tree in the middle of the plot represented the inter-specific competition. In 2010 (four years before starting the drought experiment) the 12 plots were trenched to 1 m soil depth. Below this soil depth, a layer of sandy/silty loam inhibits deeper root growth of the trees. To prevent lateral root growth and lateral water flow between the plots, the trench was lined with a plastic tarp (Grams et al. 2021). After the trees have recovered from trenching for four years, five years of drought were conducted from 2014 to 2018. The precipitation during the growing season (April to October) was excluded using automatically closing and opening throughfall exclusion roofs for half of the 12 experimental plots (6 TE plots). The second half of the plots served as the Control (6 CO plots). To eliminate the influence of small-scale factors like soil properties, each Control plot was matched with a throughfall exclusion plot (Fig. 1). Over winter, precipitation on the TE plots was allowed to replenish soil water storage (SI-Fig. 1). To ensure that the possible water uptake of the trees from outside the plots could be excluded, deuterated water was injected in deep soil layers outside the trench. The tests showed that neither the deep-rooted beech trees nor the shallow-rooted spruce trees took up labelled water. It is important to note that the experimental plots were selected randomly, taking into account the species composition. This design was chosen to ensure that the study results are as representative as possible. However, due to their species composition and other factors, a complete randomisation of the plots was not possible. Therefore, some experimental plots are adjacent to control plots. Despite this spatial proximity, all necessary preparations were made to maintain the independence of the TE and CO plots.
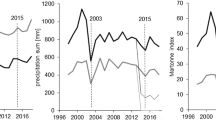
Starting in June 2019, the plots were irrigated and the roofs were permanently opened (Grams et al. 2021). Irrigation (CO + 15 mm, TE + 90 mm) in June and July 2019 and the subsequent roof opening resulted in a rapid convergence of precipitation levels on the TE and CO plots (Fig. 2). Since 2014 throughfall is different for CO and TE (SI-Fig. 1). From 1998 until 2014, the year of the throughfall exclusion, temperature and precipitation were on average 8.1 °C and 841 mm year−1, respectively. Particularly humid years in this time span were 2001 and 2002 (precipitation > 1000 mm year−1). A particularly dry year (precipitation < 700 mm year−1) was 2003. Since the start of the throughfall exclusion, temperatures have shown a slightly increasing trend (9.4 °C on average). Precipitation from 2014 onwards was slightly lower than the previous average (753 mm year−1). With the beginning of the throughfall exclusion in 2014, the reduced precipitation on the TE plots amounted to approximately a quarter of the precipitation observed on the CO plots (Fig. 2). The CO plots also faced natural droughts in 2015 and 2022, and soil water content decreased significantly during the growing seasons (SI-Fig. 1). For further information about the KROOF experimental site, plot design and dehydration technique, see (Göttlein et al. 2012; Grams et al. 2021; Häberle et al. 2012; Pretzsch et al. 1998, 2014, 2016, 2018b).
Mean annual temperature (gray line), and annual precipitation received on CO plots (black columns) and on TE plots (dark-gray columns), respectively, at the KROOF experimental site since 1998. The drought in 2003, the beginning of the experimental drought in 2014 and the end of it with onsetting irrigation and the removal of roofing in mid-2019 are highlighted by vertical lines. The additional irrigation (CO + 15 mm, TE + 90 mm) beginning in June and July 2019 are highlighted by light-gray shading stacked on top of the bar representing 2019. Meteorological data from the nearby Forest Climate Station Freising were provided by the Bavarian State Institute of Forestry
Measurements
The individual plots are similar in size, stand density, range of diameters, and mixing ratios (Table 1). On average, the CO plots have a similar plot size of 143.1 ± 34.1 m2 to the TE plots of 145.4 ± 22.4 m2. Mean diameter at breast height is close between tree species as well as between treatment groups, similarly to the mean tree heights (Table 2). However, clear differences between the tree species and especially between the different treatment groups could be observed considering the annual increment of basal area in the three periods. Here, the TE spruce and beech trees showed similar increments as the respective CO trees at the beginning of the experiment, but then during the drought the difference is clear, but not significant (iba2014-2018; spruce 14.2 ± 9.8 cm2 in CO, 4.7 ± 4.1 cm2 in TE; beech 9.0 ± 9.8 cm2 in CO, 5.1 ± 6.2 cm2 in TE). Apart from the TE plots, the site conditions in the Kranzberg forest provide almost maximum productivity for the 70-year-old spruces and the 90-year-old beeches on the experimental plots. This becomes visible by site indices of O40 according to the yield table of (Assmann and Franz 1963) for spruce, and site class I according to (Schober 1995) for beech (Fig. 3). For more information about stand and plot characteristics, as well as tree and stand growth, see (Grams et al. 2021; Pretzsch et al. 2014, 2018b, 2020a).
Current annual volume increment (m3 ha−1 year−1) at stand-level of spruce (a) and beech (b) on TE plots (solid line, filled marks) compared to the CO plots (dashed line, empty marks). The drought in 2003. the beginning of throughfall exclusion in 2014 and the end of the experimental drought-period with irrigation in mid-2019 are highlighted by vertical lines
To measure stem diameter, dendrometer bands with a resolution of 0.01 cm (Permanent-Baummessband D1, UMS GmbH, München, Deutschland) were installed at a height of 1.30 m (DBH) on each tree. Stem diameters were measured several times a year since 1998. In our study, diameters at the end of the growing season of each year from 2009 to 2022 were included. Then, radial stem growth and basal area growth were calculated for each year. Starting from the basal area, we have calculated the relative growth rate of each tree for our further analyses.
Data analysis
The relative growth rate (RGR) is a standard for describing the growth rate of an organism or a population over a certain period of time. According to plant physiology, we can assume that the increase in the dimension of a plant is more or less proportional to the already existing plant dimension. Different from absolute growth indicators, RGR allows us to compare the growth rates of different organisms or populations, even if they grow under different conditions or with different initial sizes. This makes it possible to compare growth rates of different locations or to assess the effects of environmental factors, e.g. changes in temperature or precipitation on growth rates. To evaluate ecological responses to disturbance, it can also be applied, allowing comparison of the magnitude and duration of the effects on growth. RGR is defined as the natural logarithm of the difference between the final value (e.g. biomass or size) and the initial value, divided by the time interval between the two measurements. In the case of plant growth from one year to the next, RGR can be calculated as follows, according to (Harper 1977):
where \(ln\) is the natural logarithm and \({W}_{1}\) and \({W}_{2}\) indicate plant biomass at the times \({t}_{1}\) and \({t}_{2}\). In our case \({W}_{1}\) and \({W}_{2}\) are defined as the trees basal area from last year and from the actual year divided by 1. Based on the annual RGR values from 2009 to 2020, the following models have calculated the mean periodic RGR for each individual tree of the CO and the TE plots for each of the three different experimental periods (Preperiod, Droughtperiod and Postperiod) separately. When we talk about RGR in the following text, we always refer to RGR on the basis of basal area and use the unit mm2 year−1.
A general index for assessing tree growth reaction after drought is Recovery, Rc = PostDr/Dr (Lloret et al. 2011). Recovery describes the tree growth response after the drought. This approach to measure the recovery is well suited to study singular, naturally occurring drought years. In our case, this index was less suitable for a drought period. Here, we fixed and distinguished different effects (1. general period effect and 2. pure drought stress effect due to a five-year extended drought) and then compared them with the CO. Therefore, we have chosen a post hoc test method to prove the recovery of TE spruce from the general period effect of Droughtperiod and to show that there was no recovery from the effect of throughfall exclusion.
Mixed linear models for quantification of the growth effects and the recovery after extended experimentally induced drought and subsequent irrigation (Q1)
The analysis of possible improvement in growth (recovery) after throughfall exclusion and subsequent irrigation was performed separately for spruce and beech (Q1, H01). In order to test for significant effects, we fitted a mixed linear model that describes the dependency of the RGR on the experimental period and group affiliation (CO, TE) of a tree:
The indices i, j and k represent the nesting levels of the plot, the tree within the plot and the experimental period (Preperiod, Droughtperiod, Postperiod), respectively. Droughtperiod and Postperiod are dummy variables that have the value 1 in Droughtperiod and Postperiod and 0 otherwise. The variable Treat is also a dummy variable which is 1 for all TE plots and 0 for the CO plots. As described in Eq. 1, the dependency of the RGR in the periods (Preperiod, Droughtperiod or Postperiod) and treatment groups (CO or TE) as well as the interactions of these attributes was investigated. This approach allowed us to distinguish periodic effects that occurred on the CO plots as well as on the throughfall exclusion plots from the actual effects of drought stress. In other words, the fixed effect parameters a0 to a5 contain the information required to reconstruct the expected RGR’s of the CO and TE trees in the different periods of the experiment and to test them and their differences post hoc for significance. As correlations of the observations on the plot level, and also on the tree level have to be taken into account, the model includes the random effects \({b}_{i}\) and \({b}_{ij}\) (\({b}_{i}\sim N(0, {\tau }_{1}^{2}\)), \({b}_{ij}\sim N(0, {\tau }_{2}^{2})\)). The term \({\varepsilon }_{ijk}\) represents idependently and identically distributed errors (\({\varepsilon }_{ijk}\sim N(0,{\sigma }^{2})\)). Using simultaneous post-hoc tests for general linear hypotheses (Hothorn et al. 2008), we tested whether recovery from drought stress had occurred.
Mixed linear models for Quantification of the effects of intra‑ and inter‑specific competition (Q2)
To detect the differences in growth and recovery between trees growing in inter-specific and intra-specific competition, the analyses were conducted for both tree species and treatment groups separately (Q2, H02). This was also tested for the CO trees due to the coincidental occurrence of a natural drought in 2015 during the TE period. To test for significant effects, we fitted a mixed linear model similar to the first one. The model describes the dependency of the RGR on the experimental period and group affiliation of a tree, as well as the interaction of these attributes:
The indices and the period variables are defined the same as in Eq. 1. The variable Mix is a dummy variable that is 1 for all trees that grew in inter-specific competition and 0 for those that grew in intra-specific competition. By separately applying this model to the CO and TE trees of the two species, we were able to distinguish the periodic effects that occurred within the treatment groups from the effects of the intra-specific or inter-specific competition. The procedure for determining the expected RGRs is the same as described for Eq. 1. Correlations of observations on the plot level and also on the tree level have been considered by including the random effects \({b}_{i}\) and \({b}_{ij}\) (\({b}_{i}\sim N(0, {\tau }_{1}^{2}\)), \({b}_{ij}\sim N(0, {\tau }_{2}^{2})\)). The term \({\varepsilon }_{ijk}\) represents idependently and identically distributed errors (\({\varepsilon }_{ijk}\sim N(0,{\sigma }^{2}\)).
Mixed linear models for Quantification of the shift in growth performance depending on the relative size of trees (Q3)
To determine a possible influence of the tree’s relative size on their growth reaction during the three experimental periods and to compare the growth in Droughtperiod and Postperiod regarding a possible growth improvement, the analyses for both tree species and treatment groups were conducted separately (Q3, H03). Relative tree size was chosen as an appropriate measure for a stable social tree classification throughout all observation periods. It includes on the one hand the information on the diameter, which correlates well with the social position of a tree in the stand (Pretzsch 2021), but also eliminates the absolute diameter change that the trees underwent over the three periods. By calculating the periodic diameter percentile of each tree within its species and treatment group, each tree was given a value between 0 (smallest diameter) and 1 (largest diameter) for each of the three periods. Assuming that an inert measure such as diameter is barely affected by short-term fluctuations between “top and bottom performers”, we have chosen the percentile of the periodic mean of the diameter. Hence, we considered the relative tree size in the long run. Using this concept to contrast the growth of trees of different social positions before, during, and after the drought-period, we fitted the following model that describes the dependency of the diameter increment on the experimental period and the relative tree size (percentile 0 to 1), as well as the interaction of these variables:
The indices and the period variables are defined the same as in Eqs. 1 and 2. The variable Perc is the diameter percentile that takes values between 0 and 1. A look at the variables in our model shows both: on the one hand, how the increment level changes due to the treatment in the different periods, and with regard to the interaction of diameter percentile (Perc) and the two periods (Droughtperiod and Postperiod) on the other hand, whether the slope changes significantly. This provided a way to determine the growth reaction in the three experimental periods and also made it possible to assign this reaction to trees of different relative tree size. In our model, the parameters \({a}_{0}\), \({a}_{2}\) and \({a}_{3}\) provide the information about the increment of the smallest trees during Preperiod, Droughtperiod and Postperiod. The parameters \({a}_{1}\), \({a}_{4}\) and \({a}_{5}\) reflect the growth of the trees with the highest relative tree size in the three experimental periods. Correlations of tree diameter increment on the plot level and also on the tree level have been considered by including the random effects \({b}_{i}\) and \({b}_{ij}\) (\({b}_{i}\sim N(0, {\tau }_{1}^{2}\)), \({b}_{ij}\sim N(0, {\tau }_{2}^{2})\)). The term \({\varepsilon }_{ijk}\) represents idependently and identically distributed errors (\({\varepsilon }_{ijk}\sim N(0,{\sigma }^{2}\)).
The statistical programming language R 4.0.2 (R Core Team 2020) was used for all calculations, in particular we used the lme function for regression analyses from the package nlme (Pinheiro et al. 2021) and glht from the package multcomp (Hothorn et al. 2008).
Results
Description of the reaction patterns at the stand and tree level
The annual volume increment at the stand level showed a notable decrease on the TE plots for spruce and beech (Fig. 3). Over Droughtperiod a loss of growth of 34.4 m3 ha−1 for spruce and 18,7 m3 ha−1 for beech accumulated at the stand level. This corresponds to a loss in increment of 59% for TE spruce and 42% for TE beech to their respective CO. Upon drought release due to irrigation, TE beech trees in particular showed a clear increase in volume growth. TE spruces did not show a clear upward trend in volume growth (Fig. 3a) and did not reach the level of CO. The growth of the TE beech stand almost reached the level of the CO plots after irrigation (Fig. 3b).
In the years before throughfall exclusion, the diameter increments of the trees, which were later assigned to the TE and the CO, were almost the same. Throughfall exclusion resulted in a noticeable decrease in the diameter increment of spruce and beech trees on TE plots (Fig. 4, solid line) compared to the Control (dashed line). With the beginning of the throughfall exclusion, the diameter increments of spruce fell on average steeply below the level of the Control and then stabilized at a low level of about 34% of the diameter increment of the CO group. With the start of irrigation, TE spruces noticeably recovered but remained clearly behind the CO trees. In 2022, both the TE and CO spruces experienced a natural drought. Despite this, the TE spruces showed an increase in growth, while the CO spruces showed a decrease. Consequently, the growth differences between TE and CO spruces were not significant in this year, indicating a recovery of the TE group. The diameter growth of the TE beech trees decreased to about 56% of the diameter increment of CO beeches. As a clearly recognizable acclimation, the growth recovery of TE beeches began even before irrigation started in 2019 and the diameter increment of the TE beech almost reached the level of the Control already in 2019.
Mean annual diameter increment (mm year−1) at tree-level of spruce (a) and beech (b) on the TE plots (solid line, filled marks) compared to the CO plots (dashed line, empty marks) ± CI (error bars). The drought in 2003, the beginning of throughfall exclusion in 2014 and the end of the experimental drought-period with irrigation in mid-2019 are highlighted by vertical lines and gray shading. The years of the periods that showed significant differences in the post-hoc tests are marked with asterisks
Growth reaction to extended drought and recovery after irrigation (Q1)
The regression model (Eq. 1) shows the growth reaction during and after the drought (SI-Table 1 upper part, SI = Supplementary Information; Fig. 5). The estimated mean RGR indicated a decline of tree growth on the TE plots (Fig. 5, solid lines) for both tree species and became evident as the experimental drought set in. Before the beginning of the throughfall exclusion, the periodic RGR for CO (dashed lines) and TE spruces was almost identical (Fig. 5a). In the case of beech trees (Fig. 5b), the two groups were also close during Preperiod and did not differ significantly (SI-Table 1 upper part). The TE spruces grew significantly less (p < 0.05) during the drought as well as after the irrigation (n = 95, mean 0.0038 ± 0.0012; n = 53, mean 0.0100 ± 0.0015), than the CO trees in the same periods (n = 130, mean 0.0128 ± 0.0008; n = 102, mean 0.0176 ± 0.0009). Thereby, the growth of the TE trees fell far below the level of CO, however, due to subsequent irrigation in mid-2019, spruce showed a recovery reaction in relative growth on the TE and CO plots (Fig. 5a). In the lower part of SI-Table 1, we show the results of the Simultaneous Tests for General Linear Hypotheses which were performed post hoc. Relative growth of the TE spruces deviated significantly from the CO spruces during the throughfall exclusion (ΔDroughtperiod = – 0.0092 ± 0.0027). The deviation from TE to CO was less distinct in Postperiod (ΔPostperiod = – 0.0078 ± 0.0028). Even though the relative growth of TE spruces showed a slight recovery compared to the CO spruces, this response was not significant. In relative terms, during Droughtperiod, the TE spruces lost 70% of relative growth (RGR) compared to the Control. In Postperiod, the relative growth loss was 43% compared to the Control. Beech TE trees in Droughtperiod (n = 125, mean 0.0075 ± 0.0012) had significantly reduced growth (p < 0.05) compared to the CO trees (n = 151, mean 0.0104 ± 0.0008). The relative growth rate of TE beech trees differed significantly from CO beeches during the throughfall exclusion (ΔDroughtperiod = – 0.0047 ± 0.0021) but no longer in Postperiod. Therefore, the TE beech trees showed significant recovery after irrigation compared to the CO group (ΔDroughtperiod—ΔPostperiod = 0.0049 ± 0.0013). The Control beeches showed an increase of 69% in their growth performance in 2019–2022 (Fig. 5b). Beech on the TE plots slightly decreased in RGR during the throughfall exclusion (compared to CO – 28%), increased impressively by 160% compared to its own growth performance during Droughtperiod and overtook beech on the CO plots by 11% due to its subsequent strong recovery in Postperiod. A direct comparison of spruce and beech (Fig. 5a and b) shows the obvious difference in the Preperiod between the two tree species in terms of growth performance. During Droughtperiod, spruce on the TE plots already fell below the level of beech. In contrast, the beech trees on the TE plots and the CO plots were able to catch up with spruce in growth rate due to their strong recovery responses in the four years following irrigation.
Periodic relative growth rate (RGR) ± 1 SE (gray shading) estimated by the mixed-linear model shown in Eq. 1 Spruce (a) and beech (b) on the TE plots (solid line) compared to the CO plots (dashed line). The beginning of throughfall exclusion in 2014 and the end of the drought-period with irrigation in mid-2019 are highlighted by vertical lines
Effects of intra‑ and inter‑specific competition on tree growth and recovery (Q2)
The results of our regression model fits (Eq. 2) show the intra- and inter-specific response patterns during and after the drought period, respectively (SI-Table 2; Fig. 6). The TE spruce trees showed significant decreases in RGR for Droughtperiod and Postperiod. It should be noted here that when fitting the model to the TE group alone, these effects obviously include information about experimental throughfall exclusion. The model fit for the CO trees showed the effect of Droughtperiod and Postperiod due to a significant but less pronounced decrease in RGR. In both groups, intra- and inter-specific competition showed no effect on the relative growth of spruce. Effects in Droughtperiod and Postperiod also affected the growth of the TE beech trees as shown by a significant decrease of RGR in Droughtperiod and a subsequent significant increase in Postperiod. For the CO beech trees, the model fit fixed an effect in Postperiod that was due to a significant increase in relative growth. Only beech trees of the CO group showed a significant effect in growth (p < 0.05), caused by the inter-specific competition (Fig. 6d). In Postperiod, beech mixed with spruce (Mix = BS) grew significantly less (n = 48, mean 0.0229 ± 0.0029) than beech next to beech (n = 48, mean 0.0345 ± 0.0021). Even though the model showed almost no significant differences between inter- (SM = spruce mixed, BM = beech mixed) and intra-specific (SP = spruce pure, BP = beech pure) competition, the model fits gave a good indication of trends on how the mean RGR estimated by the mixed-linear model is distributed among the groups (Fig. 6).
Periodic relative growth rate (RGR) ± 1 SE (gray shading) estimated by the mixed-linear model shown in Eq. 2. Spruce (a and b) and beech (c and d) on the TE plots (solid line) and on the CO plots (dashed line) separately. The growth of both species in inter-specific competition (black line; for spruce = SM, for beech = BM) is compared to intra-specific competition (grey line; reference for spruce = SP, reference for beech = BP). The beginning of throughfall exclusion in 2014 and the end of the drought-period with irrigation in mid-2019 are highlighted by vertical lines
Growth reaction and shift of the growth performance depending on the relative tree size during the three experimental periods (Q3)
SI-Table 3 shows the results of fitting the regression models for absolute growth response in diameter increment before and after the drought period depending on the relative tree size (Eq. 3). By applying the model fit to the TE trees of the two species, the variables Droughtperiod and Postperiod showed how the growth level changed during and after the drought as a result of the treatment. The interaction with the variable Perc provided additional information on whether the slope of the regression in Perc, and thus the increment distribution changed over the relative tree size. The growth reaction along the relative tree size in Kranzberg confirmed that TE spruce lost growth during and after extended drought stress (Fig. 7a, b and c; intercept on the y axis, solid line, filled marks). Moreover, the significant decrease in slope indicated that the trees with a high relative size were most striking in this growth reaction. This decrease became evident from the interaction of the variables Droughtperiod and Postperiod with the variable Perc (\({a}_{4}\) and \({a}_{5}\) in SI-Table 3). During Droughtperiod, the diameter increments of the TE spruce tree with the highest relative tree size (1.6571 ± 0.7249) decreased compared to Preperiod (5.5127 ± 0.7871). Similarly, during Postperiod (1.2821 ± 0.9023), the diameter increments of the tree with the highest relative tree size decreased even more compared to Preperiod. Spruce trees with a low relative tree size did not loose much growth during the drought period, slight deviations (n. s.) were compensated after the drought. Thus, the flattening of the curves for TE (Fig. 7 b and c) must mainly be attributed to a decrease in the increment of the dominant spruce trees in the stand. For CO spruce the slope of the curves, and thus the distribution of increment performance across the relative tree size remained largely the same (Fig. 7a, b and c; dashed line) and trees showed no significant deviations in Droughtperiod and in Postperiod (SI-Table 3). Beech TE showed a slight decrease (n. s.) in the growth of the individuals with a high relative tree size during extended drought stress (Fig. 7e; solid line). Beech trees with a low relative tree size also did not deviate much in growth during extended drought but increased their growth after the drought period (Fig. 7f; solid line), as evidenced by a significant increase in overall growth level (\({a}_{3}\) in SI-Table 3). That is why the TE beech tree with the lowest relative tree size showed a significant higher diameter increment in Postperiod (2.3312 ± 0.5347) than in Preperiod ( – 0.4876 ± 0.4626). The tree with the highest relative tree size increased little but significantly in diameter growth from Preperiod (3.2802 ± 0.7929) to Postperiod (3.2556 ± 0.9077; \({a}_{5}\) in SI-Table 3). The apparent flattening of the curve is mainly due to the increase in growth among the smaller beech trees in the stand. In other words, the strong recovery of TE beech from Droughtperiod to Postperiod is characterized by a slight increase in growth for individuals with a high relative tree size but mainly by a strong increase in growth of trees with a low relative tree size. This is also reflected in Fig. 7d–f. Here we have seen that in Preperiod mainly the dominant beech trees showed an increase in diameter, and many small beech trees showed a zero-growth. In Postperiod, more individuals achieved a measurable growth, the number of individuals with zero-growth decreased, and especially the TE beech trees with a low relative tree size became "high performers” in the stand. In contrast, many CO beech trees with low relative tree size remained at the bottom end of diameter increment. The CO beech tree with the lowest relative tree size showed a significant deviation from Preperiod ( – 0.1823 ± 0.4793) to Postperiod (1.0244 ± 0.3883; \({a}_{3}\) in SI-Table 3). Thereby, the growth level of all CO beeches increased. However, the slope of the curves and thus the distribution of the growth performance along the relative tree size did not change for CO.
Mean periodic diameter increment (mm year−1 period.−1) plotted over the percentile of stem diameter in the three experimental periods (Preperiod 2009–2013. Droughtperiod 2014–2018 and Postperiod 2019–2022) for spruce (a, b, c) and beech (d, e, f), on the TE plots (solid line, filled marks) and the CO plots (dashed line, empty marks) ± 1 SE (gray shading)
Finally, our results can be summarized as follows: During 5 years of experimentally drought, beech suffered less than spruce in tree growth and recovered faster than spruce trees under controlled irrigation after the drought period. Spruce trees subjected to experimental drought continued to deteriorate compared to Control even after irrigation. During the drought period, but also after irrigation, we did not detect significant differences in growth due to throughfall exclusion, depending on intra- or inter-specific competition, but were able to see trends. Due to a shift in the growth performance along the relative tree size in the stand, growth reductions of the previously best-performing trees were partly compensated by an increased growth of the trees with lower relative tree size.
Discussion
Growth reaction and acclimation to extended drought and recovery after irrigation (Q1)
A five-year extended drought caused significant growth losses in both, spruce and beech (Fig. 5), but after irrigation spruce showed a delayed recovery (n.s.) while beech showed a strong and early recovery in the first two years (SI-Table 1, Fig. 4b). This may be interpreted as better acclimation of beech to drought, and as an indicator for serious damage on spruce, as described in recent studies (Arend et al. 2021; Bottero et al. 2021). In 2022, both CO and TE spruce and beech trees experienced a natural drought (see materials and methods section, SI-Fig. 1) which did not reduce the growth of TE spruce, while TE beech growth declined continuously, following the first year of recovery, as did CO spruce and beech trees (Fig. 4a). In summary, our null hypothesis H01 was rejected as we showed species-specific growth and recovery from drought upon irrigation for spruce and beech. Our insights on the different drought response of spruce and beech align with recent research that highlighted the role of nutrient regime, drought frequency, and hydraulic conditions in previous and subsequent years as critical factors in the drought response of these species (Schmied et al. 2023). This study linking higher drought frequency with increased resistance and resilience of spruce and beech provides an additional perspective that complements our focus on the effects of a prolonged five-year drought.
Kannenberg et al. (2019b), Kannenberg et al. (2019a) and Ruehr et al. (2019) discussed that drought stress responses may persist beyond the drought itself. Ovenden et al. (2021) showed that those responses did not solely negatively affected growth if longer post-drought periods were chosen or, as in our study, another drought event followed. Based on the different hydraulic functions introduced by McDowell et al. (2008), spruce (isohydric) and beech (anisohydric), two abiotic mechanisms i.e. carbon starvation and hydraulic failure are discussed as the causes of growth decline and tree mortality under drought (Adams et al. 2017; Hartmann 2011; McDowell et al. 2008, 2019; Sevanto et al. 2014), which also can occur in combination (Adams et al. 2017) and possibly even relate to each other (Kannenberg et al. 2019b).
Spruce, with its more conservative isohydric strategy, reduces acute water loss by closing its stomata (Vogel et al. 2017; Zavadilová et al. 2023). This mechanism can cause carbon starvation and occurs more frequently in gymnosperms than in angiosperms, which can also be associated with the hydraulic vulnerability of the xylem, thereby playing a role in reducing hydraulic function (Adams et al. 2017) and may also inhibit the long-term growth of new supply structures. It is possible that spruce thereby was unable to recover quickly when water became available again (Choat et al. 2018; Wu et al. 2018).
But delayed recovery after disturbance does not necessarily imply impaired function, and may also be a sign of acclimation reactions at the expense of growth (Gessler et al. 2020). Possible mechanisms of spruce may be hydraulic acclimations of leavess and branches, due to an increased hydraulic safety and decreased productivity (Tomasella et al. 2018). Such negative growth legacies were also discussed by Gessler et al. (2020) as possible positive acclimation responses and thus as long-term survival strategies of the tree with regard to future drought events. Also acclimations of root growth (Brunner et al. 2015; Gao et al. 2021), or crown morphology might fall under such mechanisms. We assume that the Norway spruce TE trees benefit from their xeromorphic needles and bark built in the years of the experimental drought period. Those needle cohorts are still maintained several years after the end of the experimental drought, whereas in the case of beech the newly built leaves and branches do not have this morphological acclimation (Pretzsch et al. 2021). In addition, the leaf area reduction, shortening and xeromorphic anatomy of needles (Gebhardt et al. 2023) and xylem vessels with small diameters (Levionnois et al. 2021) built by the dried-out Norway spruces may have reduced the risk of embolism in the natural drought period 2022.
Beech follows the more "risky" anisohydric strategy regarding water consumption by leaving the stomata open, even when the water supply decreases, which can lead to hydraulic failure already after short periods of intense stress (Hesse et al. 2023; Petrík et al. 2022). Hydraulic failure can occur during a drought when the tree looses more water through its leaves than can be replenished by the roots (Brodribb et al. 2020; Choat et al. 2012).
In line with the anisohydric strategy, Nikolova et al. (2009) showed that under drought, beech was able to assimilate more carbon and invest it in stem and root growth than spruce. Our research confirms the advantage of beech in terms of relative stem growth not only under mild to moderate drought but also under severe and prolonged (five years) drought. This could be a possible explanation for the specific recovery reaction of beech and also the interesting detail that its recovery was already indicated during the drought period, even before the opening of the roof and the start of irrigation (Fig. 4b). Beech suffered less within the experimental drought period, probably took up deeper water than spruce, reduced probably less the xylem vessel diameters and thus is more prone to xylem failure under repeated drought events (Petit et al. 2022). The increased die-off of spruce on some TE plots during the drought period and the associated reduced stand density may also have contributed to the strong recovery of beech (Pretzsch et al. 2020a). The mortality of neighbors allowed more soil water to be used by the remaining individuals (SI-Fig. 1). This may have caused the two systems of beech and spruce to diverge, as spruce no longer absorbed water and the soil actually became wetter (especially in the plots where trees died). SI-Fig. 1 indicates that from 2017 onwards, the soil water content increased as well on the TE plots. The beeches may have shown fewer stress symptoms during the experimental drought, possibly due to the spruces no longer taking up water or the beech trees rooting deeper.
In addition, also more indirect drought effects such as a change in the composition of the microbiome may be considered as acclimation (Gao et al. 2021). As the stands have not been thinned for more than 20 years silvicultural interventions may be excluded as possible causes for a modified resistance and recovery under drought stress (Pretzsch 2022).
It should be mentioned that Fig. 5 shows an increased growth rate for both beech groups from 2019 onwards. This increase, also for CO, is likely due to a coincidental accumulation of relatively dry years during the experimental Droughtperiod 2014–2018 (see the temperature to precipitation ratio in Fig. 2). In consequence, both groups experienced increased growth from 2019. Both spruce groups, including CO, suffered significant growth losses between 2014 and 2018, so a recovery phase was required after 2019 to compensate for these losses. One possible explanation could be that beech is benefiting from spruce’s competitive weakness in the medium to long term. While spruce struggles in the Postperiod, beech could gain an advantage by using nutrients and water that would have otherwise been consumed by spruce. This could lead to an increase in growth for beech, which would indicate a shift in growth from spruce to beech. From this perspective, the naturally dry conditions between 2014 and 2018 may have resulted in less resource consumption by spruce, potentially favoring beech due to increased resource availability.
Effects of intra‑ and inter‑specific competition on tree growth and recovery (Q2)
Spruce and beech tended to grow more in inter-specific than in intra-specific competition (n. s.) during a five-year extended drought (Fig. 6a and c) and tended to grow less in inter-specific competition than in the intra-specific competition under CO conditions with only beech showing significant differences (Fig. 6b and d). We observed a trend that for spruce the mixture appears to be more important during recovery after the extended drought stress than during the actual drought period itself. Thus, our null hypothesis H02 was clearly rejected for the TE trees, but we showed tendencies of specific effects of competition (inter- vs. intra-specific) on the recovery from drought which was in addition tree-species-specific.
Previous studies have addressed species-specific stress reactions of trees in monocultures compared to mixed stands (Bello et al. 2019; Pardos et al. 2021; Schwarz and Bauhus 2020; Vergarechea et al. 2021). In many cases, an improved stress response has been confirmed by an increased growth in tree mixture (Ammer 2019; Pretzsch et al. 2013). Such benefits of mixture may depend on stand density, site conditions (Lévesque et al. 2016; Steckel et al. 2020b), species combination (Grossiord et al. 2014; Grossiord 2018; Steckel et al. 2020a), and mixture proportion, among other factors (Pretzsch et al. 2020b), and may be reversed to a disadvantage when the stress factor is diminished (Pretzsch et al. 2015a). Temporal complementarity in water uptake may explain the slight reduction of stress reaction in Kranzberg for TE spruce and beech in mixture (Rötzer et al. 2017), which could benefit both tree species by avoiding simultaneous water demand of conspecifics that occurs in the pure stand (Thurm et al. 2016). Relatively, spruce benefited slightly more from the mixture than beech during the drought (Fig. 6a and c), as it started transpiration earlier and used soil water first under inter-specific competition, which was partially replenished in winter (Pretzsch et al. 2020a). By the end of the throughfall-exclusion and start of irrigation, soil water was able to recover from mid-2019, about three months earlier than during the five-year drought. Thus, in relative terms, spruce was better able to exploit its advantage of earlier water uptake in the following spring. The shallow fine roots of the spruces probably enabled them to access winter precipitation, but also irrigation in summer 2019, earlier than the deeper-rooted beeches. At least in the mixed zone of plots, Zwetsloot and Bauerle (2021) were able to verify that there was increased production of fine roots, whereas drought drastically reduced fine root production by spruce in the species-pure zone. Thus, the species complementarity could explain why spruce in the SM constellation continued to benefit more than beech in the BM constellation during and after the drought, even though these differences were not significant and could only be assumed visually. To prevent the spruces against bark beetle attack we started in 2015 annually spraying of the spruce crowns and stem surfaces with the contact insecticide Karate Forst liquid using the canopy crane. Without this measure, the drought damages on the plots may had been worse as drought can increase the susceptibility of spruce to insect attacks (Netherer et al. 2019). However, many studies (Jactel 2017; Skatulla 1989; Wermelinger 2004) show that tree species mixing can increase the resistance of Norway spruce against biotic disturbances e.g., bark beetle (Ips typographus L.) or gregarious spruce sawfly (Pristiphora abietina (Christ.) (Hym., Tenthredinidae)). And according to the growth-differentiation balance hypothesis (Matyssek et al. 2012) the continuous drought on the TE plots may have increased the trees’ resistance against biotic disturbances (Nardi et al. 2023).
Growth reaction and shift of the growth performance depending on the relative tree size of trees during the three experimental periods (Q3)
In Kranzberg spruce trees with high relative tree size were most affected by growth losses during and after an extended drought, while beech trees with a high relative tree size showed just a slight decrease in growth during the extended drought. Spruce trees with low relative tree size did not loose much growth at all, while beech trees with low relative tree size increased their growth after the drought period. Now we can better assign the basic growth patterns we checked with H01 to the relative tree size within the two tree species. The strong decrease in the growth of spruce was mainly caused by the dominant trees, while the strong recovery of beech was mainly caused by a strong growth increase of trees with low relative tree size (Fig. 7a–f). Our null hypothesis H03 was distinctly rejected as we showed specific effects of the relative tree size (social position) on growth performance during and recovery from drought which also was tree-species-specific.
Most studies dealing with growth reactions under drought stress are limited to the evaluation of the dominant trees in a stand. Consistent with recent studies like Ding et al. (2017); Pretzsch (2021) and Zang et al. (2012) we have shown that the growth reaction to drought can vary depending on tree size (diameter percentile). In line with other studies like Grote et al. (2016); Pretzsch et al. (2018b) and Pretzsch (2022) we showed that there was a significant shift in growth performance that favoured the smaller trees in the stand which has led to a more size-symmetric distribution of increment across diameter classes. The causal explanation might be that cooler microclimate and less transpiration demand can be expected in the shade-tolerant species, protecting from heat damage and decreasing drought stress (Grote et al. 2016). In dry years, small trees may benefit indirectly from the reduced water consumption of the tall trees, which are more exposed to heat, drought and stomata closure. Thus the growth partitioning may become more size-symmetric in dry years (Schwinning and Weiner 1998; Wichmann 2001) and on dry sites (Pretzsch and Dieler 2011). Under moist conditions, after ending of the experimental drought, large trees can make again use of their preferential access to light, increase their growth and water consumption and thus reduce the growth of smaller neighbors (Grote et al. 2016). This enables a change in competition mode from a more size-symmetric competition during drought to size-asymmetric competition under sufficient soil water supply as also described by Zang et al. (2012). Pretzsch and Biber (2010) and Pretzsch et al. (2022) showed that this may result in more heterogeneous stands under dry conditions or in periods with drought events and more homogeneous stands under moist conditions where the large trees can pre-empt the light and outcompete small neighbors. We observed that this shift occurred not only during drought stress but also in the post-drought period, where mostly the larger spruces lost growth performance, while mainly the small-sized beeches benefited. This is in itself an interesting species-specific redistribution of growth. With regard to the results from H01, we saw how the initial growth depression of spruce two years after the drought period occurred primarily in the large trees, and the remarkably good growth in the natural drought year of 2022 was achieved by the smaller trees (Fig. 4a). We also saw that some of the lost growth potential may be transferred to the small sized beech trees after the drought. Therefore, we concluded that in a spruce-beech stand under extended drought stress, the sequence of stress reaction and recovery reaction may lead to a reversal of the previous growth distribution among individuals and thus may have an effect on the vertical structure of future forest stands. Such a relative advantage of small trees results in lower mortality and an accumulation of previously subdominant trees in the understory (Pretzsch et al. 2018b). Our results are in line with other studies (Chakraborty et al. 2021; Grote et al. 2016) and show that water availability can have a great influence on the structure and dynamics of forest stands. This is not only important for dry ecosystems but is already a common issue in temperate regions and is supposed to get more important under a climate with longer drought periods.
Functional and structural diversification as risk prevention
Under the experimental drought stress, in the phase of stress release, and also under the subsequent natural drought the two species reacted very differently (Figs. 5 and 6). We also found very different reactions on drought stress and stress release in different size classes (Fig. 7). This suggests a functional diversification that may stabilize the function and growth of forest stands (Bauhus et al. 2017; Larsen 1995; Pardos et al. 2021). However, Haberstroh and Werner (2022) recently reported that under extreme drought any synergistic inter-specific interactions may fail.
A silvicultural approach that promotes structural and genetic diversity may not achieve maximum productivity under normal conditions, i.e. without disturbances (Assmann 1970). However, with increasing frequency of disturbances the resistance and resilience by structural and genetic diversification may achieve advantageous productivity, stability and other ecosystem functions and services (Biber et al. 2015; del Río et al. 2022; Dieler et al. 2017). Thus silvicultural may prefer prescription that favour tree species mixture and structural layering, e.g. selection forest systems with 3 or 4 species as risk prevention (Pretzsch and Zenner 2017; Schütz 2001).
The recent commentary by Ulrich and Grossiord (2023) highlights the complexity of tree responses to drought, particularly regarding species-specific strategies for stomatal regulation along the iso-anisohydric continuum. They point out that while anisohydric species are often considered as more drought resistant, this may not always be the case, and that even anisohydric species with higher embolism resistance and hydraulic safety margins may have higher mortality rates than isohydric species that co-occur. Therefore, it is possible that classification of tree species along the iso-anisohydric continuum is not sufficient to fully capture drought vulnerability.
Moreover, Ulrich and Grossiord (2023) describe the potential role of water availability in shaping the structure and dynamics of forest ecosystems. They point out that even in temperate regions, water availability may become a critical factor, which could affect our forest composition. This evaluation is consistent with our findings on the different responses of spruce and beech to extended drought.
This adds complexity to our understanding of functional diversification and species-specific drought response and underscores the need for a nuanced approach to predicting future forest dynamics under changing climatic conditions.
Considerations and perspectives
In our study, we discussed acclimation in TE beech before irrigation, and recovery in TE beech and the small TE spruce trees after irrigation. A possible, but in our study not considered cause for a recovery reaction might be the sudden availability of nutrients that could not be dissolved in soil water during the drought and suddenly became plant available with the onset of precipitation and irrigation (Gessler et al. 2017). Such mechanism and the question why this then primarily favored the beech trees would have to be further investigated.
Furthermore, it was not certain if the growth reactions were actual acclimation and recovery response or a simple shift of released resources from more suffering individuals to favored individuals. In this scenario, the delayed pattern of benefiting could be explained by the need for favored individuals to access the released resources first.
It should be measured whether crucial parts of hydraulic conduits may occluded by gas emboli and if the trees could not compensate by building up new tissue (Brodribb et al. 2010). The remaining portion of water-conducting tissue after a drought might also determine how well a tree recovers (Bréda et al. 2006; Choat et al. 2018). A possible advantage of the anisohydric strategy is that beech can still assimilate carbon during drought to form new conductive vessels. Later this may become an additional risk, as these vessels can increase the tree’s vulnerability to recurrent droughts (Gessler et al. 2020). Deeper research on the microscale, such as analysis of wood anatomy, would provide further insight.
Besides the limitations discussed, we want to mention that the design of our study did not allow for complete randomisation of the experimental plots due to the species composition. Some control and treatment plots were close to each other, which might affect the generalisability of our results. Despite our efforts to randomly select experimental plots taking into account species composition, future research should aim for even better randomisation to minimise possible bias.
Especially for the study of acclimation effects under drought, long-term experimental manipulation studies like those in Kranzberg are essential and may be useful to complement studies along natural precipitation gradients that taken place before. Together, the Kranzberg throughfall exclusion experiment and the associated precipitation gradient from the KROOF project may allow us to distinguish drought acclimation effects from long-term adaptations to generally dry site conditions. To date, such field manipulation studies at an ecosystem level as the KROOF experiment are still very rare, but provide new and important evidence on how spruce and beech might respond to future climate. Even if such large-scale experiments are very costly, they contribute significantly to the understanding of trees responses in complex environmental networks.
Conclusion
An important highlight and new insight of our study is a more comprehensive picture of recovery reactions in the growth of mature spruce and beech after a long-term drought for the latitudes of Central Europe. With five years of drought followed by four years of recovery, a complete growth trajectory consisting of pre-, during-, and post-periods has been quantified. We considered not only possible differences in growth reactions in intra- and inter-specific competition of the trees but also differences between dominant and small trees.
In summary, growth reactions to drought are specific to tree species and mixture can influence this reaction, even though in our case only tendencies were observed. Furthermore, the structuring of small to dominant trees has a clear influence on the response of the stand as a whole. A redistribution pattern has emerged in favour of the smaller trees and at the expense of the dominant trees.
According to our results, we assume that spruce is significantly more drought-sensitive than beech during and also after prolonged droughts. Due to the superior recovery response of beech, the generally high productivity of spruce may face increased competitive pressure. This could particularly occur if consecutive drought years became more frequent and the Beech could play out its ability to recover. In conclusion, this could lead to a sustained decrease in the proportion of spruce in future forest ecosystems and its importance in silviculture could decline accordingly. However, its surprisingly good radial growth in the natural drought year 2022 indicated that spruce still has potential due to acclimation, apart from the general recovery. Nevertheless, the volume growth of the TE spruce trees remains far below the CO trees and the O40 site index line (Fig. 3a), indicating that spruce growth is still limited. Summarizing we conclude that spruce and beech can acclimate to a changed climate with an associated change in water supply even during prolonged drought stress.
Mixing economically valuable spruce with more drought-resistant tree species like beech might balance the needs of economics and ecology. The frequently reported drought stress relief of spruce in mixture is an argument for a transition to mixed forest stands. Size-symmetric partitioning patterns along the relative tree size due to drought could result in a more even growth distribution and in the long term more heterogeneous stand structures under recurring and persistent drought stress. This may cause and accelerate changes in the distribution, species composition, and vertical structure of forests.
Author contributions statement
FM and HP conceptuated the study. FM evaluated the data and wrote the paper. HP and KP initiated the project where the study at hand is embedded in. TR, KP, and HP developed and established the experimental design. HP, PB, EU and TR contributed to evaluating the data and writing the manuscript. HP, PB, EU, TR and KP revised the manuscript and contributed to the interpretation of the results.
Availability of data and material
The materials described in the manuscript including all relevant raw data will be freely available upon request from the corresponding author.
Code availability
The R-codes described in the manuscript and designed for statistical analysis are freely available on request from the corresponding author.
References
Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhäusser SM, Tissue DT et al (2017) A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat Ecol Evolut 1:1285–1291
Aldea J, Ruiz-Peinado R, del Río M, Pretzsch H, Heym M, Brazaitis G et al (2021) Species stratification and weather conditions drive tree growth in Scots pine and Norway spruce mixed stands along Europe. Forest Ecol Manag 481:118697
Allen CD, Breshears DD (1998) Drought-induced shift of a forest-woodland ecotone: rapid landscape response to climate variation. Proc Natl Acad Sci USA 95:14839–14842
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell NG, Vennetier M et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol Manag 259:660–684
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:art129
Ammer C (2019) Diversity and forest productivity in a changing climate. New Phytol 221:50–66
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA 109:233–237
Arend M, Link RM, Patthey R, Hoch G, Schuldt B, Kahmen A (2021) Rapid hydraulic collapse as cause of drought-induced mortality in conifers. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2025251118
Assmann E (1970) The principles of forest yield study: Studies in the organic production, structure, increment and yield of forest stands. Pergamon Press
Assmann E, Franz F (1963) Vorläufige Fichten-Ertragstafel für Bayern, S. 125
Aussenac R, Bergeron Y, Gravel D, Drobyshev I (2019) Interactions among trees: A key element in the stabilising effect of species diversity on forest growth. Funct Ecol 33:360–367
Bauhus J, Forrester DI, Gardiner BA, Jactel H, Vallejo R, Pretzsch H (2017) Ecological Stability of Mixed-Species Forests. In: Pretzsch H, Forrester DI, Bauhus J (eds) Mixed-Species Forests. Springer, Berlin, pp 337–382
Bello J, Vallet P, Perot T, Balandier P, Seigner V, Perret S et al (2019) How do mixing tree species and stand density affect seasonal radial growth during drought events? Forest Ecol Manag 432:436–445
Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ (2015) Larger trees suffer most during drought in forests worldwide. Nat Plants. https://doi.org/10.1038/nplants.2015.139
Biber P, Borges J, Moshammer R, Barreiro S, Botequim B, Brodrechtová Y et al (2015) How sensitive are ecosystem services in european forest landscapes to silvicultural treatment? Forests 6:1666–1695
Bosela M, Tobin B, Šebeň V, Petráš R, Larocque GR (2015) Different mixtures of Norway spruce, silver fir, and European beech modify competitive interactions in central European mature mixed forests. Can J for Res 45:1577–1586
Bosela M, Tumajer J, Cienciala E, Dobor L, Kulla L, Marčiš P et al (2021) Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. Sci Total Environ 752:141794
Bottero A, Forrester DI, Cailleret M, Kohnle U, Gessler A, Michel D et al (2021) Growth resistance and resilience of mixed silver fir and Norway spruce forests in central Europe: Contrasting responses to mild and severe droughts. Glob Change Biol 27:4403–4419
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann for Sci 63:625–644
Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG et al (2005) Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102:15144–15148
Brodribb TJ, Bowman DMJS, Nichols S, Delzon S, Burlett R (2010) Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol 188:533–542
Brodribb TJ, Powers JS, Cochard H, Choat B (2020) Hanging by a thread? Forests and drought. Science 368:261–266
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6:547
Chakraborty T, Reif A, Matzarakis A, Saha S (2021) How does radial growth of water-stressed populations of european beech (Fagus sylvatica L.) trees vary under multiple drought events? Forests 12:129
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R et al (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558:531–539
Ciais P, Reichstein M, Viovy N, Granier A, Ogèe J, Allard V et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Dale VH, Joyce LA, McNulty S, Neilson RP, Ayres MP, Flannigan MD et al (2001) Climate change and forest disturbances. Science 51:723
del Río M, Condés S, Pretzsch H (2014a) Analyzing size-symmetric vs. size-asymmetric and intra- vs. inter-specific competition in beech (Fagus sylvatica L.) mixed stands. For Ecol Manag 325:90–98
del Río M, Schütze G, Pretzsch H (2014b) Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol 16:166–176
del Río M, Pretzsch H, Ruiz-Peinado R, Jactel H, Coll L, Löf M et al (2022) Emerging stability of forest productivity by mixing two species buffers temperature destabilizing effect. J Appl Ecol 59:2730–2741
Dieler J, Uhl E, Biber P, Müller J, Rötzer T, Pretzsch H (2017) Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. Forstwissenschaftliches Centralblatt 136:739–766
Ding H, Pretzsch H, Schütze G, Rötzer T (2017) Size-dependence of tree growth response to drought for Norway spruce and European beech individuals in monospecific and mixed-species stands. Plant Biol (stuttg) 19:709–719
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht: 203 Tabellen. 6th edn. Ulmer, XXII, 1333 S
Englund G, Cooper SD (2003) Scale effects and extrapolation in ecological experiments. In Advances in ecological research: Vol. 33. H. Caswell (ed). Academic Press, pp. 161–213
Gao D, Joseph J, Werner RA, Brunner I, Zuercher A, Hug C et al (2021) Drought alters the carbon footprint of trees in soils-tracking the spatio-temporal fate of C-13-labelled assimilates in the soil of an old-growth pine forest. Glob Change Biol 27:2491–2506
Gebhardt T, Hesse BD, Hikino K, Kolovrat K, Hafner BD, Grams TEE, Häberle K-H (2023) Repeated summer drought changes the radial xylem sap flow profile in mature Norway spruce but not in European beech. Agric for Meteorol 329:109285
Gessler A, Schaub M, McDowell NG (2017) The role of nutrients in drought-induced tree mortality and recovery. New Phytol 214:513–520
Gessler A, Bottero A, Marshall JD, Arend M (2020) The way back: recovery of trees from drought and its implication for acclimation. New Phytol. https://doi.org/10.1111/nph.16703
Göttlein A, Baumgarten M, Dieler J (2012) Site Conditions and Tree-Internal Nutrient Partitioning in Mature European Beech and Norway Spruce at the Kranzberger Forst. In: Matyssek R, Schnyder H, Oßwald W, Ernst D, Munch JC, Pretzsch H (eds) Growth and Defence in Plants. Springer, Berlin, pp 193–211
Grams TEE, Hesse BD, Gebhardt T, Weikl F, Rötzer T, Kovacs B et al (2021) The Kroof experiment: realization and efficacy of a recurrent drought experiment plus recovery in a beech/spruce forest. Ecosphere 12:11
Grossiord C (2018) Having the right neighbors: how tree species diversity modulates drought impacts on forests. New Phytol 228:42–49
Grossiord C, Granier A, Ratcliffe S, Bouriaud O, Bruelheide H, Checko E et al (2014) Tree diversity does not always improve resistance of forest ecosystems to drought. Proc Natl Acad Sci USA 111:14812–14815
Grote R, Gessler A, Hommel R, Poschenrieder W, Priesack E (2016) Importance of tree height and social position for drought-related stress on tree growth and mortality. Trees 30:1467–1482
Gupta A, Rico-Medina A, Cano-Delgado AI (2020) The physiology of plant responses to drought. Science 368:266–269
Häberle K-H, Weigt RB, Nikolova PS, Reiter IM, Cermak J, Wieser G et al (2012) Case Study “Kranzberger Forst”: Growth and Defence in European Beech (Fagus sylvatica L.) and Norway Spruce (Picea abies (L.) Karst.). In Growth and Defence in Plants. Matyssek R, Schnyder H, Oßwald W, Ernst D, Munch JC, Pretzsch H (Eds). Springer, Berlin, pp. 243–271
Haberstroh S, Werner C (2022) The role of species interactions for forest resilience to drought. Plant Biol (stuttg) 24:1098–1107
Harper JL (1977) Population biology of plants. 3rd edn. Academic Press, 892 p
Hartmann H (2011) Will a 385 million year-struggle for light become a struggle for water and for carbon? - How trees may cope with more frequent climate change-type drought events. Glob Change Biol 17:642–655
Hartmann H, Moura CF, Anderegg WRL, Ruehr NK, Salmon Y, Allen CD et al (2018) Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol 218:15–28
Hesse BD, Goisser M, Hartmann H, Grams TEE (2019) Repeated summer drought delays sugar export from the leaf and impairs phloem transport in mature beech. Tree Physiol 39:192–200
Hesse BD, Gebhardt T, Hafner BD, Hikino K, Reitsam A, Gigl M et al (2023) Physiological recovery of tree water relations upon drought release-response of mature beech and spruce after five years of recurrent summer drought. Tree Physiol 43:522–538
Hlásny T, Trombik J, Bošeľa M, Merganič J, Marušák R, Šebeň V et al (2017) Climatic drivers of forest productivity in Central Europe. Agric for Meteorol 234–235:258–273
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biomet J Biometrische Zeitschrift 50:346–363
Ibáñez I, Acharya K, Juno E, Karounos C, Lee BR, McCollum C et al (2019) Forest resilience under global environmental change: Do we have the information we need? A systematic review. PLoS ONE 14:e0222207
IPCC (Ed) (2014) Climate change 2014: Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team RK Pachauri and LA Meyer (Eds.)]. Intergovernmental Panel on Climate Change, 151 p
Jactel H (2017) Tree diversity drives forest stand resistance to natural disturbances. Curr for Rep 3:223–243
Kannenberg SA, Phillips RP (2020) Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol 40:259–271
Kannenberg SA, Novick KA, Alexander MR, Maxwell JT, Moore DJP, Phillips RP, Anderegg WRL (2019a) Linking drought legacy effects across scales: From leaves to tree rings to ecosystems. Glob Change Biol 25:2978–2992
Kannenberg SA, Novick KA, Phillips RP (2019b) Anisohydric behavior linked to persistent hydraulic damage and delayed drought recovery across seven North American tree species. New Phytol 222:1862–1872
Kolář T, Čermák P, Trnka M, Žid T, Rybníček M (2017) Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in Central Europe. Agric for Meteorol 239:24–33
Krejza J, Cienciala E, Světlík J, Bellan M, Noyer E, Horáček P et al (2021) Evidence of climate-induced stress of Norway spruce along elevation gradient preceding the current dieback in Central Europe. Trees 35:103–119
Krupkova L, Havrankova K, Krejza J, Sedlak P, Marek MV (2019) Impact of water scarcity on spruce and beech forests. J for Res 30:899–909
Larsen JB (1995) Ecological stability of forests and sustainable silviculture. For Ecol Manag 73:85–96
Latte N, Lebourgeois FF, Claessens H (2016) Growth partitioning within beech trees (Fagus sylvatica L.) varies in response to summer heat waves and related droughts. Trees 30:189–201
Lebourgeois FF, Eberlé P, Mérian P, Seynave I (2014) Social status-mediated tree-ring responses to climate of Abies alba and Fagus sylvatica shift in importance with increasing stand basal area. For Ecol Manag 328:209–218
Leuzinger S, Zotz G, Asshoff R, Körner C (2005) Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol 25:641–650
Lévesque M, Saurer M, Siegwolf RTW, Eilmann B, Brang P, Bugmann H, Rigling A (2013) Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob Change Biol 19:3184–3199
Lévesque M, Walthert L, Weber P (2016) Soil nutrients influence growth response of temperate tree species to drought. J Ecol 104:377–387
Levionnois S, Jansen S, Wandji RT, Beauchene J, Ziegler C, Coste S et al (2021) Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytol 229:1453–1466
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J et al (2010) Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manag 259:698–709
Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120:1909–1920
Matyssek R, Koricheva J, Schnyder H, Ernst D, Munch JC, Osswald W, Pretzsch H (2012) The Balance Between Resource Sequestration and Retention: A challenge in plant science. In growth and defence in plants resource allocation at multiple scales prologue. Luettge U (Ed), pp. 3–24
McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb NS, Kolb TE et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McDowell NG, Brodribb TJ, Nardini A (2019) Hydraulics in the 21st century. New Phytol 224:537–542
McGregor IR, Helcoski R, Kunert N, Tepley AJ, Gonzalez-Akre EB, Herrmann V et al (2021) Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol 231:601–616
Nardi D, Jactel H, Pagot E, Samalens J-C, Marini L (2023) Drought and stand susceptibility to attacks by the European spruce bark beetle: A remote sensing approach. Agri for Entomol 25:119–129
Netherer S, Panassiti B, Pennerstorfer J, Matthews B (2019) Acute drought is an important driver of bark beetle infestation in Austrian Norway Spruce Stands. Front for Global Change 2:265
Nikolova PS, Raspe S, Andersen CP, Mainiero R, Blaschke H, Matyssek R, Häberle K-H (2009) Effects of the extreme drought in 2003 on soil respiration in a mixed forest. Eur J for Res 128:87–98
Ovenden TS, Perks MP, Clarke T-K, Mencuccini M, Jump AS (2021) Life after recovery: Increased resolution of forest resilience assessment sheds new light on post-drought compensatory growth and recovery dynamics. J Ecol 109:3157–3170
Pardos M, del Río M, Pretzsch H, Jactel H, Bielak K, Bravo F et al (2021) The greater resilience of mixed forests to drought mainly depends on their composition: Analysis along a climate gradient across Europe. For Ecol Manag 481:118687
Petit G, Zambonini D, Hesse BD, Haeberle K-H (2022) No xylem phenotypic plasticity in mature Picea abies and Fagus sylvatica trees after 5 years of throughfall precipitation exclusion. Glob Change Biol 28:4668–4683
Petrík P, Zavadilová I, Šigut L, Kowalska N, Petek-Petrik A, Szatniewska J et al (2022) Impact of Environmental Conditions and Seasonality on Ecosystem Transpiration and Evapotranspiration Partitioning (T/ET Ratio) of Pure European Beech Forest. Water 14:3015
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2021) nlme: Linear and nonlinear mixed effects models
Pretzsch H (2022) The emergent past: past natural and human disturbances of trees can reduce their present resistance to drought stress. Eur J for Res 141:87–104
Pretzsch H, Biber P (2010) Size-symmetric versus size-asymmetric competition and growth partitioning among trees in forest stands along an ecological gradient in central Europe. Can J for Res 40:370–384
Pretzsch H, Dieler J (2011) The dependency of the size-growth relationship of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in forest stands on long-term site conditions, drought events, and ozone stress. Trees 25:355–369
Pretzsch H, Zenner EK (2017) Toward managing mixed-species stands: from parametrization to prescription. For Ecosyst 4:43
Pretzsch H, Kahn M, Grote R (1998) Die Fichten-Buchen-Mischbestände des Sonderforschungsbereiches „Wachstum oder Parasitenabwehr?“ im Kranzberger Forst. Eur J for Res 117:241–257
Pretzsch H, Block J, Dieler J, Dong PH, Kohnle U, Nagel J et al (2010) Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Ann for Sci 67:712
Pretzsch H, Schütze G, Uhl E (2013) Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant Biol 15:483–495
Pretzsch H, Rötzer T, Matyssek R, Grams TEE, Häberle K-H, Pritsch K et al (2014) Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: from reaction pattern to mechanism. Trees 28:1305–1321
Pretzsch H, del Río M, Ammer C, Avdagić A, Barbeito I, Bielak K et al (2015a) Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur J for Res 134:927–947
Pretzsch H, Forrester DI, Rötzer T (2015b) Representation of species mixing in forest growth models. A review and perspective. Ecol Modell 313:276–292
Pretzsch H, Bauerle TL, Häberle K-H, Matyssek R, Schütze G, Rötzer T (2016) Tree diameter growth after root trenching in a mature mixed stand of Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]). Trees 30:1761–1773
Pretzsch H, Forrester DI, Bauhus J (eds) (2017) Mixed-Species Forests. Springer, Berlin
Pretzsch H, del Río M, Biber P, Arcangeli C, Bielak K, Brang P et al (2018a) Maintenance of long-term experiments for unique insights into forest growth dynamics and trends: review and perspectives. Eur J for Res 138:1–21
Pretzsch H, Schütze G, Biber P (2018b) Drought can favour the growth of small in relation to tall trees in mature stands of Norway spruce and European beech. For Ecosyst 5:227
Pretzsch H, del Río M (2020) Density regulation of mixed and mono-specific forest stands as a continuum: a new concept based on species-specific coefficients for density equivalence and density modification. For Int J for Res 93:1–15
Pretzsch H, Grams TEE, Häberle K-H, Pritsch K, Bauerle TL, Rötzer T (2020a) Growth and mortality of Norway spruce and European beech in monospecific and mixed-species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees 18:93
Pretzsch H, Hilmers T, Uhl E, Bielak K, Bosela M, del Río M et al (2020b) European beech stem diameter grows better in mixed than in mono-specific stands at the edge of its distribution in mountain forests. Eur J for Res 1:1–19
Pretzsch H, Motte F, Grams TEE, Prtisch K, Rötzer T, Hesse BD, Häberle K-H (2021) Fichten und Buchen bei Trockenheit im Rein-und Mischbestand. AFZ/Der Wald, 12–15
Pretzsch H, Bravo-Oviedo A, Hilmers T, Ruiz-Peinado R, Coll L, Löf M et al (2022) With increasing site quality asymmetric competition and mortality reduces Scots pine (Pinus sylvestris L.) stand structuring across Europe. For Ecol Manag 520:120365
Pretzsch H (2021) The social drift of trees. Consequence for growth trend detection, stand dynamics, and silviculture. Eur J for Res 176:9
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing
Rais A, Uhl E, van de Kuilen J-WG, Pretzsch H (2021) Short-term reaction of European beech stem taper due to weather extremes. For Ecol Manag 480:118653
Rohner B, Kumar S, Liechti K, Gessler A, Ferretti M (2021) Tree vitality indicators revealed a rapid response of beech forests to the 2018 drought. Ecol Indicat 120:106903
Rötzer T, Häberle K-H, Kallenbach C, Matyssek R, Schütze G, Pretzsch H (2017) Tree species and size drive water consumption of beech/spruce forests - a simulation study highlighting growth under water limitation. Plant Soil 418:337–356
Ruehr NK, Grote R, Mayr S, Arneth A (2019) Beyond the extreme: recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol 39:1285–1299
Rukh S, Poschenrieder W, Heym M, Pretzsch H (2020) Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level. Forests 11:639
Schmied G, Hilmers T, Mellert K-H, Uhl E, Buness V, Ambs D et al (2023) Nutrient regime modulates drought response patterns of three temperate tree species. Sci Total Environ 868:161601
Schober R (1995) Ertragstafeln wichtiger Baumarten bei verschiedener Durchforstung. 4th edn. Sauerländer, 166 p
Schuldt B, Buras A, Arend M, Vitasse Y, Beierkuhnlein C, Damm A et al (2020) A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl Ecol 45:86–103
Schütz J-P (2001) Opportunities and strategies of transforming regular forests to irregular forests. For Ecol Manag 151:87–94
Schwarz JA, Bauhus J (2020) Benefits of mixtures on growth performance of silver Fir (Abies alba) and European Beech (Fagus sylvatica) increase with tree size without reducing drought tolerance. Front for Glob Change. https://doi.org/10.3389/ffgc.2019.00079
Schwinning S, Weiner J (1998) Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 113:447–455
Seidl R, Thom D, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G et al (2017) Forest disturbances under climate change. Nat Clim Chang 7:395–402
Sevanto S, McDowell NG, Dickman LT, Pangle RE, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161
Skatulla U (1989) Eine Methode zur erfolgreichen Bekämpfung der kleinen Fichtenblattwespe, Pristiphora abietina (Christ.) (Hym., Tenthredinidae). Anzeiger Für Schädlingskunde Pflanzenschutz Umweltschutz 62:156–157
Steckel MS, del Río M, Heym M, Aldea J, Bielak K, Brazaitis G et al (2020a) Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) - Site water supply and fertility modify the mixing effect. For Ecol Manag 461:117908
Steckel MS, Moser WK, del Río M, Pretzsch H (2020b) Implications of reduced stand density on tree growth and drought susceptibility: a study of three species under varying climate. Forests 11:627
Stovall AEL, Shugart HH, Yang X (2019) Tree height explains mortality risk during an intense drought. Nat Commun. https://doi.org/10.1038/s41467-019-12380-6
Thurm EA, Uhl E, Pretzsch H (2016) Mixture reduces climate sensitivity of Douglas-fir stern growth. For Ecol Manag 376:205–220
Tomasella M, Beikircher B, Häberle K-H, Hesse BD, Kallenbach C, Matyssek R, Mayr S (2018) Acclimation of branch and leaf hydraulics in adult Fagus sylvatica and Picea abies in a forest through-fall exclusion experiment. Tree Physiol 38:198–211
Trouvé R, Bontemps J-D, Collet C, Seynave I, Lebourgeois FF (2014) Growth partitioning in forest stands is affected by stand density and summer drought in sessile oak and Douglas-fir. For Ecol Manag 334:358–368
Ulrich DEM, Grossiord C (2023) Faster drought recovery in anisohydric beech compared with isohydric spruce. Tree Physiol 43:517–521
Vergarechea M, Calama R, Pretzsch H, Alday JG, del Río M (2021) Short- and long-term growth response to climate in mixed and monospecific forests of Pinus pinea and Pinus pinaster. Eur J for Res 140:387–402
Vogel T, Votrubova J, Dohnal M, Dusek J (2017) A Simple representation of plant water storage effects in coupled soil water flow and transpiration stream modeling. Vadose Zone J 16:1–10
Walthert L, Ganthaler A, Mayr S, Saurer M, Waldner P, Walser M et al (2021) From the comfort zone to crown dieback: Sequence of physiological stress thresholds in mature European beech trees across progressive drought. Sci Total Environ 753:141792
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manag 202:67–82
Wichmann L (2001) Annual variations in competition symmetry in even-aged sitka spruce. Ann Bot 88:145–151
Wu X, Liu H, Li X, Ciais P, Babst F, Guo W et al (2018) Differentiating drought legacy effects on vegetation growth over the temperate Northern Hemisphere. Glob Change Biol 24:504–516
Zang CS, Pretzsch H, Rothe A (2012) Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 26:557–569
Zavadilová I, Szatniewska J, Petrík P, Mauer O, Pokorný R, Stojanović M (2023) Sap flow and growth response of Norway spruce under long-term partial rainfall exclusion at low altitude. Front Plant Sci 14:1089706
Zhao M, Running SW (2010) Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science (new York, NY) 329:940–943
Zwetsloot MJ, Bauerle TL (2021) Repetitive seasonal drought causes substantial species-specific shifts in fine-root longevity and spatio-temporal production patterns in mature temperate forest trees. New Phytol. https://doi.org/10.1111/nph.17432
Acknowledgements
The authors wish to thank the German Science Foundation (Deutsche Forschungsgesellschaft) for funding the project “From near-death back to life: Mixed stands of spruce and beech under drought stress and stress recovery. From pattern to process” (# DFG PR 292/22-1). We would also like to thank the Bavarian State Ministry for Nutrition, Agriculture, and Forestry for funding the project, “W047” (# GZ: 7831-28160-2018). We further thank the Bayerische Staatsforsten (BaySF) for supporting the establishment of the plots and the Bavarian State Ministry for Nutrition, Agriculture, and Forestry for permanent support of the project “W07 Long-term experimental plots for forest growth and yield research” (# 7831-22209-2013). Thanks also go to Prof. Dr. Thorsten E. E. Grams, Dr. Karl-Heinz Häberle, Dr. Julia Schmack and Michael Heym for their constructive criticism. For the soil water contend data we would like to thank Kyohsuke Hikino and the Professorship for Land Surface- Atmosphere Interactions, Ecophysiology of Plants, TUM School of Life Sciences. Thanks are also due to the Bavarian State Institute of Forestry (LWF) for providing the meteorological data from the nearby forest climate station Kranzberg Forest/Freising.
Funding
Open Access funding enabled and organized by Projekt DEAL. German Science Foundation (Deutsche Forschungsgemeinschaft); funding the project “From near-death back to life: Mixed stands of spruce and beech under drought stress and stress recovery. From pattern to process” (# DFG PR 292/22-1). Bavarian State Ministry for Nutrition, Agriculture, and Forestry; funding the project, “W047 “ (# GZ: 7831-28,160-2018). Bavarian State Ministry for Nutrition, Agriculture, and Forestry; supporting the project “W07 Long-term experimental plots for forest growth and yield research” (# 7831-22,209-2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Kajimoto.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motte, F., Rötzer, T., Biber, P. et al. Growth of European beech recovered faster than that of Norway spruce after a five-year experimental drought in a mixed forest stand. Trees 37, 1695–1715 (2023). https://doi.org/10.1007/s00468-023-02453-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02453-x