Abstract
Background
Severe chronic kidney disease (CKD) in children and young adults has shown to be associated with abnormal brain development, which may contribute to neurocognitive impairments. We aimed to investigate risk factors for neurocognitive impairment and investigate the relation with structural brain abnormalities in young severe CKD patients.
Methods
This cross-sectional study includes 28 patients with severe CKD (eGFR < 30), aged 8–30 years (median 18.5 years), on different treatment modalities (pre-dialysis [n = 8], dialysis [n = 8], transplanted [n = 12]). We assessed neurocognitive functioning using a comprehensive test battery and brain structure by magnetic resonance imaging metrics of brain volume and white matter integrity (fractional anisotropy [FA] and mean diffusivity [MD] measured with diffusion tensor imaging). Multivariate regression and mediation analyses were performed between clinical CKD parameters, brain structure, and neurocognitive outcome.
Results
A combination of risk factors (e.g., longer time since kidney transplantation, longer dialysis duration and late CKD onset) was significantly associated with lower intelligence and/or worse processing speed and working memory. Lower FA in a cluster of white matter tracts was associated with lower intelligence and mediated the relation between clinical risk factors and lower intelligence.
Conclusions
Young severe CKD patients with a prolonged duration of kidney replacement therapy, either dialysis or transplantation are at particular risk for impairments in intelligence, processing speed, and working memory. Disrupted white matter integrity may importantly contribute to these neurocognitive impairments. Prospective, longitudinal studies are needed to elucidate the mechanisms involved in CKD and treatment that affect white matter integrity and neurocognitive outcome in young patients.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Young patients with advanced stages of chronic kidney disease (CKD) are at particular risk for school and vocational problems, which have partly been attributed to impaired neurocognitive functioning associated with CKD [1,2,3]. CKD-associated neurocognitive dysfunction may be expressed in impaired processing speed, attention, memory and executive functioning [2, 4]. Likewise, severe CKD is suggested to be associated with abnormal brain development (e.g., due to exposure to dialysis treatment, hypertension, and neurotoxic and vasoactive immunosuppressive therapy after kidney replacement therapy (KRT) [5,6,7,8,9]), which may play an important role in the development of these neurocognitive impairments. Recently, we showed that children and young adults with severe CKD are at risk for widespread disruption of white matter integrity and regional loss of subcortical volume (i.e., in nucleus accumbens) [10], but the association between such abnormalities and neurocognitive functioning in young patients with severe CKD has remained largely unexplored.
In adults and elderly with advanced stages of CKD, both lower white matter integrity and smaller brain volumes have been related to impairments in global neurocognitive functioning, attention, and executive functioning [11,12,13,14,15,16]. In children and young adults with CKD, studies have reported on the association between structural brain abnormalities and neurocognitive functioning in children with mild to moderate stages of CKD [17, 18] or patients up to 25 years old with mild to severe stages of CKD [19]. These studies yielded inconsistent findings and did not find clear evidence for an association between CKD-associated brain abnormalities and neurocognitive functioning. To date, the potential relevance of brain volume and white matter integrity for neurocognitive functioning has not been investigated in children and young adults with advanced CKD stages receiving KRT. Given the vulnerability of the developing brain for diffuse impact [20, 21] and the potential impact on daily life functioning, better understanding of the relation between severe CKD-associated brain abnormalities and neurocognitive functioning in young patients is of great importance.
The current study aims to explore the potential role of structural brain abnormalities in the relationship between advanced CKD, different treatment modalities, and neurocognitive functioning (i.e., intelligence and specific neurocognitive functions).
Methods
Participants
Twenty-eight CKD patients with severe CKD were recruited from the Amsterdam University Medical Center (Amsterdam UMC, n = 25); Erasmus Medical Center, the Netherlands (n = 1); and the University Hospital Antwerp, Belgium (n = 2). Inclusion criteria were as follows: (1) CKD stage 4–5 (estimated glomerular filtration rate [eGFR] < 30 ml/min/1.73 m2) on conservative therapy, on peritoneal or hemodialysis or patients having received a kidney transplant at least 2 years prior to enrollment (to ensure stable kidney function), and (2) aged between 8 and 30 years. Exclusion criteria were as follows: (1) previously established severe intellectual impairment; (2) insufficient mastery of the Dutch language; (3) primary sensory disorder (hearing or vision impairments); (4) established skull or brain abnormalities not related to CKD; or (5) co-existing disease with primary or secondary central nervous system involvement interfering with the impact of CKD.
Treatment subgroups
The following treatment subgroups were distinguished: (1) a pre-dialysis group (n = 8) with eGFR < 30 ml/min/1.73 m2 on conservative treatment at time of assessment; (2) a dialysis group (n = 8); and (3) a transplanted group (n = 12) of patients with a stable, functioning kidney graft for at least 2 years and eGFR > 30 ml/min/1.73 m2.
Measurements
Socio-demographic
Socio-demographic characteristics (i.e., age, sex, parental educational level) were collected using a custom-made questionnaire assessed through the online KLIK portal [22]. Parental educational level was divided into three categories: (1) low education (primary education, lower vocational education, lower and middle general secondary education); (2) middle education (middle vocational education, higher secondary education, pre-university education); and (3) high education (higher vocational education, university) [23].
Clinical CKD parameters and previous MRI outcomes
Clinical CKD parameters were extracted from the patient’s medical file and included: age at diagnosis of severe CKD, current eGFR, duration of severe CKD (ratio of time interval between date at which eGFR < 30 ml/min/1.73 m2 and assessment date to calendar age, expressed as % of life), dialysis duration (ratio of dialysis duration to calendar age, i.e., % of life), and time since successful transplantation (ratio of time interval between date of transplantation and eGFR > 30 ml/min/1.73 m2 and assessment date to calendar age, i.e., % of life).
Brain structure was assessed by MRI, using diffusion tensor imaging with tract-based spatial statistics to determine white matter integrity and using segmentation-based volumetric analysis on T1 scans to determine brain volumes. Details on the MRI acquisition and preprocessing are described elsewhere [10]. For this study, only brain measures with observed differences between our CKD patients (n of MRI patient sample = 23) and a control group were used. This involved white matter integrity in a large cluster of white matter tracts (i.e., lower fractional anisotropy [FA; value ranging from 0.0 to 1.0] and higher mean diffusivity [MD], mainly in the inferior frontal occipital fasciculus [IFOF], anterior thalamic radiation [ATR], superior longitudinal fasciculus [SLF], cortical spinal tract [CST], and uncinate fasciculus) and smaller volume in a subcortical gray matter structure (i.e., nucleus accumbens).
Neurocognitive functioning
A short form of the Wechsler (Adult) Intelligence Scale (WISC/WAIS, third edition) was used to estimate age-standardized full-scale intelligence quotient (eFSIQ; M = 100, SD = 15) and interpreted based on clinical interpretation guidelines [24]. A comprehensive neurocognitive test battery was administered to assess a wide range of specific neurocognitive functions (see Table 1). For these neurocognitive tests, age-standardized scaled scores were calculated (e.g., by comparing the patients’ individual score to normative data) for each participant and subsequently transformed to z-scores where lower scores correspond to worse performance.
To reduce the number of neurocognitive outcome measures, data reduction was performed using principal component analyses with Varimax rotation on the age-standardized z-scores derived from the neurocognitive tasks (see Supplement 1 for specifications). The principal component analyses constructed five domains, accounting for 76% of explained variance. Based on factor loadings the neurocognitive domains were labeled as follows: (1) processing speed and working memory; (2) fluency; (3) verbal memory; (4) processing speed, switch, and control; (5) switching.
Procedure
The study protocol was approved by the Medical Ethics Committee of the Amsterdam UMC (#NL61708.018.17), and all procedures were performed according to the Declaration of Helsinki. Eligible patients were first approached by their treating physician. Interested participants were contacted by a member of the research team and received a comprehensive information letter. After 2 weeks, potential candidates were re-contacted by telephone to answer any possible remaining questions. Written informed consent was obtained from legal guardians (for children aged < 16 years old) and/or children or young adults aged ≥ 12 years.
The 30-min MRI scanning and 90-min neurocognitive assessments (administered in a dedicated assessment room by a trained neuropsychologist using standardized instructions) were performed on the same day and took place at the Amsterdam UMC. One week earlier, participants and/or parents of participants < 18 years old completed the online questionnaires.
Statistical analyses
Socio-demographic and clinical characteristics
Statistical analyses were performed using SPSS 26.0 (IBM Corp., 2019). Independent and dependent variables were tested for normality and screened for outliers (± 3 interquartile ranges below/above the lower or upper quartile), which were rescaled using Winsorizing [25]. Missing data in the neurocognitive outcomes (M = 2%, range: 0–7%) were replaced by multiple imputation [26]. All treatment subgroups were compared with each other on demographic characteristics (age, sex, and parental educational level) and clinical CKD parameters using analysis of variance (ANOVA), to enable subgroup comparisons on outcome variable. Multivariate regression models were checked for multicollinearity. Socio-demographic characteristics (i.e., age, sex and parental educational level) were added as covariates to all analyses.
Neurocognitive functioning
First, univariate linear regression analyses were performed for each clinical CKD parameter (age at diagnosis of severe CKD, current eGFR, severe CKD duration, dialysis duration, and time since successful transplantation). Then, the relationships between pre-selected clinical CKD parameters (with p < 0.20 in univariate analyses) and neurocognition were investigated using multivariate linear regressions with backward elimination (in order to account for suppressor effects [25], criterion for removal: p > 0.10). The neurocognitive outcome measurements were used as dependent variables (eFSIQ and neurocognitive domains) in multivariate linear regressions. Additionally, explorative analyses using ANOVA tested possible differences in neurocognitive functioning between treatment subgroups (pre-dialysis group, dialysis group, transplanted group).
Brain structure and neurocognitive functioning
We investigated the relation between brain structure and neurocognitive functioning using several steps. First, we aimed to determine the clusters of white matter tracts that are related to neurocognitive outcomes with observed sensitivity to CKD (i.e., neurocognitive outcomes that were significantly related to clinical CKD parameters in the analyses described above). This was performed using voxel-wise regression between these neurocognitive outcomes and FA within a cluster of white matter tracts that has previously been shown to be affected by CKD in this sample [10]. A voxel-wise approach was chosen to analyze white matter integrity, as it was expected that the impact of CKD on white matter integrity is diffuse and not limited to pre-specified regions of interest. FSL 5.0.11 randomize was used, where threshold-free cluster enhancement and family-wise error corrected p-values accounted for multiple testing. Any resulting clusters of white matter tracts with a significant relationship between FA and neurocognitive outcome were selected as regions of interest (ROI) for subsequent multivariate analysis.
Next, multivariate analyses on the relation between brain structure and neurocognitive functioning were performed in SPSS. Univariate linear regression analyses were performed for each aspect of brain structure with previously determined sensitivity for CKD in this sample (i.e., FA and MD in ROIs as determined in the previous step, and subcortical volume of the nucleus accumbens as determined in previous work on this sample [10]) on the neurocognitive outcomes with observed sensitivity to CKD using the multivariate regressions with backward elimination. From these univariate analyses, pre-selected aspects of brain structure (p < 0.20) were entered in multivariable linear regression models with backward elimination on neurocognitive outcomes with observed sensitivity to CKD. Finally, the potentially mediating role of brain structure in the relation between CKD and neurocognitive functioning was explored using mediation models with PROCESS version 4.0 using 5000 bootstrap samples [27].
All statistical testing was two-sided and alpha was set at 0.05. Cohen’s d effect sizes are reported where appropriate and were interpreted as small (d < 0.5), medium (0.5 ≥ d < 0.8), or large (d ≥ 0.8) [28].
Results
Socio-demographic and clinical characteristics
Age at diagnosis was significantly higher in the dialysis group than in the transplanted group (p = 0.010, d = 1.34). Treatment subgroups differed significantly in terms of eGFR, blood urea, and time since successful transplantation (Table 2).
Neurocognitive functioning in severe CKD patients
Univariate analyses in the total CKD group on eFSIQ showed p-values < 0.20 for dialysis duration and time since successful transplantation (Supplement 2). The final multivariate regression analysis revealed that longer time since successful kidney transplantation was significantly related to lower eFSIQ (Table 3), with parental educational level and dialysis duration included in the model as non-significant predictors.
Univariate analyses on processing speed and working memory showed p-values < 0.20 for age at CKD diagnosis and dialysis duration. The final multivariate regression analysis revealed that higher age at CKD diagnosis and longer dialysis duration were significantly related to worse processing speed and working memory.
Univariate analyses on verbal memory showed p-values < 0.20 for duration of severe CKD and dialysis duration. The final multivariate regression analysis on verbal memory was not significant. Univariate analyses for other neurocognitive domains were not significant.
Neurocognitive functioning in CKD treatment groups
Explorative analyses comparing treatment subgroups on neurocognitive functioning were not significant (Table 4). Based on clinical interpretation guidelines, exploratory visual inspection of eFSIQ and processing speed and working memory data shows that the pre-dialysis group scored in the high/above average range and both the dialysis and transplanted group scored in the low-average range. Therefore, we also explored differences in eFSIQ and processing speed and working memory domain between the group not receiving kidney replacement therapy (non-KRT: the pre-dialysis group) and the groups receiving kidney replacement therapy (KRT: dialysis and transplanted group combined) (see Supplement 3). These exploratory analyses showed that the non-KRT group had significant higher eFSIQ than the KRT group (p = 0.030, d = 0.99). The comparison on processing speed and working memory was not significant (p = 0.157, d = − 0.94).
Association between brain structure and neurocognitive functioning in severe CKD patients
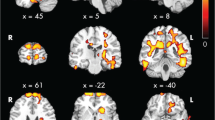
Regional associations between FA and neurocognitive functions with observed effects of CKD revealed a cluster of white matter tracts in the whole CKD group (complete MRI and neurocognitive data, n = 23), in which lower FA was associated with lower eFSIQ (Fig. 1). This cluster of tracts involves the SLF, CST, ATR, and IFOF. No regional associations between FA and processing speed and working memory were observed. The cluster of white matter tracts relating to eFSIQ in CKD patients was selected for further analyses investigating the relation between brain structure and neurocognitive functioning.
Regional associations between FA in cluster affected by CKD and eFSIQ using threshold-free cluster enhancement correction in TBSS, showing a significant positive correlation between eFSIQ and white matter integrity in CKD patients (displayed in red–yellow, n = 23, sociodemographic characteristics were included in the model as covariates). Image is illustrated the following coordinates: (x = 26, y = − 7, z = 20) and shows the whole brain skeleton (at FA > 0.3, in green), overlaid on standard MNI 152 1-mm T1 brain. Significant group differences are “thickened” towards the full width of the white matter tract to increase visualization. The scatterplot shows the association between eFSIQ and FA in the cluster that is associated with eFSIQ in the CKD sample. Abbreviations: FA, fractional anisotropy; L, left
Univariate analyses on eFSIQ showed p-values < 0.20 for FA in the selected cluster of white matter tracts (Supplement 4). The final multivariate regression analyses revealed that lower FA in the selected cluster of white matter tracts and lower parental education level were significantly related to lower eFSIQ. Univariate analyses on processing speed and working memory showed p-values < 0.20 for FA in the selected cluster of white matter tracts. The final multivariate regressions analyses revealed that older age and lower parental educational level were significantly related to worse processing speed and working memory performance (Table 5).
Mediation analysis revealed that lower FA in the selected cluster of white matter tracts significantly mediated the relation between time since successful kidney transplantation and eFSIQ (95% confidence interval = − 0.92 to − 0.15; Fig. 2).
Mediation model testing the impact of FA in the cluster of white matter tracts associated with eFSIQ in CKD patients in the association between time since successful kidney transplantation (% of life) and eFSIQ. Age, sex, and parental educational level were added as covariates to mediation analyses. Illustration of the indirect (paths A and B), direct (path C) and total effects (path C′), showing that the effect of time since successful kidney transplantation on eFSIQ is mediated by FA in the cluster of white matter tracts associated with eFSIQ. Abbreviations: FA = fractional anisotropy; B = unstandardized regression coefficient; eFSIQ = estimated full-scale intelligence quotient; SE = standard error
Discussion
In this study, we explored the impact of a selection of clinical risk factors on neurocognitive functioning and the relation with structural brain abnormalities in children and young adults with severe CKD. We found that greater exposure to dialysis, longer time since transplantation and later onset of severe CKD are risk factors of worse neurocognitive outcomes (i.e., intelligence and/or processing speed and working memory). In addition, the association between longer time since transplantation and lower intelligence was mediated by regional disruption of white matter integrity. The results of this study suggest that the occurrence of white matter abnormalities in young patients with advanced stages of CKD may play an instrumental role in the development of neurocognitive impairment.
We found no evidence for a direct impact of low eGFR or duration of severe CKD on neurocognitive functioning. Although planned analyses initially showed no group differences between treatment subgroups, exploratory analysis suggests that patients on KRT (transplanted and dialysis patients) may have worse neurocognitive outcome than pre-KRT patients with an eGFR below 30 ml/min/1.73 m2. This gives rise to the hypothesis that (a history of) prolonged duration of KRT may be more strongly related to neurocognitive functioning than exposure to uremic toxins. This hypothesis remains to be further investigated, but is in line with previous studies showing that neurocognitive functioning of transplanted children is impaired in comparison to healthy peers [2] and that neurocognitive functions are sensitive to the impact of dialysis in both pediatric and adult patients [2, 4, 29]. Our finding that greater exposure to the state of kidney transplantation is associated with lower intelligence contrasts with outcomes from adult CKD studies, which show that neurocognitive functioning of adults with a well-functioning kidney graft is not impaired relative to healthy controls [4]. Together, these potentially worrying findings would imply that the course of CKD and transplantation may have a different and more negative impact on neurocognitive functioning of young patients compared to adult patients. Our finding that patients with an older age at severe CKD diagnosis have worse neurocognitive functioning regarding processing speed and working memory is surprising and needs to be confirmed in future studies. However, it could be speculated that early reorganization (i.e., neuroplasticity) in the younger brain may (partly) compensate the initial impact of CKD on processing speed and working memory. Finally, no evidence was found for an impact of severe CKD on other neurocognitive domains (e.g., fluency, verbal memory, cognitive control and switching), which somewhat contrasts with previous studies in children and adult patients [2, 4]. Although our study may have lacked power to detect subtle effects of severe CKD on specific neurocognitive domains, this discrepancy may also be explained by differences in assessment methodology which may subvert direct comparisons between study outcomes. Furthermore, previous claims that children with CKD are at risk for executive function problems have often been based on patient and proxy report questionnaires [2], which are known to measure a different construct than performance-based assessments of executive functioning [30].
Our analyses also suggest that disrupted white matter integrity in specific tracts (i.e., SLF, CST, ATR, and IFOF) is related to lower intelligence in our severe CKD patients. This partly contrasts with a previous study in children with mild to moderate stages of CKD where no evidence for an association between CKD-related white matter integrity and neurocognitive functioning was found [18], suggesting that abnormal white matter integrity may have stronger relevance to neurocognitive functioning in more advanced stages of CKD than in milder stages of CKD. This implies that advanced CKD may be associated with abnormal development of white matter tracts, possibly causing less efficient information processing within brain neural networks that are essential for higher-order functions, such as intelligence [31, 32]. Indeed, lower white matter integrity has previously been linked to worse global neurocognitive functioning in adults with CKD [12, 14]. Importantly, we found a mediating role for white matter disruption in the relation between time since transplantation and lower intelligence. This finding may suggest that CKD-related disease mechanisms disrupt white matter integrity in young patients, subsequently contributing to neurocognitive impairments.
To our knowledge, this is the first study to report a mediating role of white matter integrity in neurocognitive outcome of CKD patients and this observation awaits replication in longitudinal studies. The association with time since transplantation may reflect that the initial impact of CKD on the developing brain and intelligence may continue after transplantation. Additionally, from this finding, it could be speculated that factors post-transplantation (e.g., impaired microcirculation, neurotoxic and vasoactive immunosuppressive therapy) may have further detrimental effects on brain structure and neurocognitive functioning [7,8,9]. The idea that an impaired microcirculation (e.g., due to hypertension or vaso-active medication) is implicated in the development of white matter abnormalities and associated neurocognitive impairments post-transplantation is consistent with previous work showing that the burden of cardiovascular disease increases with longer time since transplantation [33] and that cardiovascular risk factors are both related to lower white matter integrity and neurocognitive problems [34, 35].
Taken together, we argue that our findings reflect a complex pathogenesis of CKD-related brain abnormalities that may comprise both disease and treatment (i.e., transplantation and dialysis) -related factors. These factors may lead to white matter disruption and subsequent neurocognitive impairment, which may not fully recover after transplantation.
Limitations and strengths
First, we acknowledge our small sample size. Therefore, cautious interpretation of our findings is necessary. We encourage further investigation of neurocognitive functioning and underlying neuropathology in dialysis and transplanted patients using prospective, long-term longitudinal designs with multivariate repeated measurements within individual patients to increase rigor of the observations and to follow the course of CKD throughout disease and treatment stages. We cannot exclude that other factors may have influenced neurocognitive development in conjunction with CKD, such as prematurity, perinatal events, and genomic disorders that are prevalent co-occurring diagnoses in this population and have previously been associated with neurocognitive impairments [36]. It is important that future research in larger cohorts take into account the potential roles of co-morbidities and clinical complications of CKD (e.g., hypertension, prematurity, prolonged severe acidosis, proteinuria, disease etiology). A second limitation is the lack of a matched control group. Although we compared neurocognitive functioning of our CKD patients to normative data, it is necessary to replicate these findings and compare patients to a matched healthy control group. A third limitation is the heterogeneity of the sample in terms of socio-demographic and illness characteristics. Sociodemographic variables were included as covariates in statistical analyses to take possible confounding factors into account, to partly account for heterogeneity. This study also has several strengths. It is the first study to focus on the association between CKD-related white matter abnormalities and neurocognitive functioning in a representative sample of young Dutch-speaking patients with advanced CKD on different treatment modes, using an extensive neuropsychological test battery, relate this to brain structure (using advanced quantitative analyses), and perform mediation analyses. Thereby, this study contributes to our understanding of the disease mechanisms and neural mechanisms that underlie neurocognitive impairment in young patients with severe CKD.
Clinical implications and future directions
Our findings highlight the importance for clinicians to be aware of neurocognitive problems in children and young adults with advanced CKD, in particular patients who have a complex medical history of prolonged time on KRT. As compared to pre-KRT patients, patients on dialysis as well as transplanted patients seem to be at risk for neurocognitive impairment. When clinicians have concerns on neurocognitive functioning of a child or young adult with CKD, it is recommended to conduct neuropsychological assessment to identify possible neurocognitive impairments to determine which patients may benefit from (neuro)psychological support.
This is the first study to indicate a mediating role of CKD-associated brain white matter abnormalities in the development of neurocognitive impairments in young severe CKD patients, which may provide a potential target for the development of neurocognitive rehabilitation strategies. Physical exercise is suggested to indirectly improve neurocognitive functioning in healthy adolescents and young adults through a possible beneficial impact on white matter integrity (i.e., through the increase of neurotrophic factors and cerebral blood flow) [37,38,39,40]. This suggests an opportunity for physical exercise as a neurocognitive rehabilitation strategy, although effectiveness in clinical populations remains to be determined [41].
Finally, it is important that prospective longitudinal studies further determine the effects of dialysis and kidney transplantation on neurocognitive functioning and underlying neural mechanisms (e.g., using functional brain connectivity and/or measuring biomarkers of neuroaxonal injury) as well as investigating the impact of worse neurocognitive functioning on health-related quality of life and adaptive functioning.
Abbreviations
- ATR:
-
Anterior thalamic radiation
- ANOVA:
-
Analysis of variance
- CKD:
-
Chronic kidney disease
- CST:
-
Corticospinal tract
- eFSIQ:
-
Estimated age-standardized full-scale intelligence quotient
- eGFR:
-
Estimated glomerular filtration rate
- FA:
-
Fractional anisotropy
- IFOF:
-
Inferior fronto-occipital fasciculus
- MD:
-
Mean diffusivity
- MRI:
-
Magnetic resonance imaging
- Non-KRT:
-
Non-kidney replacement therapy
- KRT:
-
Kidney replacement therapy
- ROI:
-
Region of interest
- SLF:
-
Superior longitudinal fasciculus
References
Groothoff JW, Grootenhuis M, Dommerholt A, Gruppen MP, Offringa M, Heymans HS (2002) Impaired cognition and schooling in adults with end stage renal disease since childhood. Arch Dis Child 87:380–385
Chen K, Didsbury M, van Zwieten A, Howell M, Kim S, Tong A, Howard K, Nassar N, Barton B, Lah S, Lorenzo J, Strippoli G, Palmer S, Teixeira-Pinto A, Mackie F, McTaggart S, Walker A, Kara T, Craig JC, Wong G (2018) Neurocognitive and educational outcomes in children and adolescents with CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol 13:387–397. https://doi.org/10.2215/cjn.09650917
Duquette PJ, Hooper SR, Wetherington CE, Icard PF, Gipson DS (2007) Brief report: intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol 32:1011–1017. https://doi.org/10.1093/jpepsy/jsm036
Vanderlinden JA, Ross-White A, Holden R, Shamseddin MK, Day A, Boyd JG (2019) Quantifying cognitive dysfunction across the spectrum of end-stage kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 24:5–16. https://doi.org/10.1111/nep.13448
Moodalbail DG, Reiser KA, Detre JA, Schultz RT, Herrington JD, Davatzikos C, Doshi JJ, Erus G, Liu HS, Radcliffe J, Furth SL, Hooper SR (2013) Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol 8:1429–1448. https://doi.org/10.2215/cjn.11601112
Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, Terasaki T (2002) Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem 83:57–66. https://doi.org/10.1046/j.1471-4159.2002.01108.x
Parvex P, Pinsk M, Bell LE, O’Gorman AM, Patenaude YG, Gupta IR (2001) Reversible encephalopathy associated with tacrolimus in pediatric renal transplants. Pediatr Nephrol 16:537–542. https://doi.org/10.1007/s004670100602
Tantisattamo E, Molnar MZ, Ho BT, Reddy UG, Dafoe DC, Ichii H, Ferrey AJ, Hanna RM, Kalantar-Zadeh K, Amin A (2020) Approach and management of hypertension after kidney transplantation. Front Med (Lausanne) 7:229. https://doi.org/10.3389/fmed.2020.00229
Van Sandwijk MS, Ten Berge IJ, Majoie CB, Caan MW, De Sonneville LM, Van Gool WA, Bemelman FJ (2016) Cognitive changes in chronic kidney disease and after transplantation. Transplantation 100:734–742. https://doi.org/10.1097/tp.0000000000000968
Lijdsman S, Königs M, van Sandwijk MS, Bouts AH, van Hoeck K, de Jong H, Engelen M, Oosterlaan J, Bemelman FJ, Oostrom KJ, Groothoff JW (2021) Structural brain abnormalities in children and young adults with severe chronic kidney disease. Pediatr Nephrol 37:1125–1136. https://doi.org/10.1007/s00467-021-05276-5
Zhang LJ, Wen J, Ni L, Zhong J, Liang X, Zheng G, Lu GM (2013) Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis 28:647–654. https://doi.org/10.1007/s11011-013-9438-7
Findlay MD, Dawson J, Dickie DA, Forbes KP, McGlynn D, Quinn T, Mark PB (2019) Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol 30:147–158. https://doi.org/10.1681/asn.2018050462
Mu J, Chen T, Li P, Ding D, Ma X, Zhang M, Liu J (2018) Altered white matter microstructure mediates the relationship between hemoglobin levels and cognitive control deficits in end-stage renal disease patients. Hum Brain Mapp 39:4766–4775. https://doi.org/10.1002/hbm.24321
Yin Y, Li M, Li C, Ma X, Yan J, Wang T, Fu S, Hua K, Wu Y, Zhan W, Jiang G (2018) Reduced white matter integrity with cognitive impairments in end stage renal disease. Front Psychiatry 9:143. https://doi.org/10.3389/fpsyt.2018.00143
Yeh YC, Kuo YT, Huang MF, Hwang SJ, Tsai JC, Kuo MC, Chen CS (2019) Association of brain white matter lesions and atrophy with cognitive function in chronic kidney disease. Int J Geriatr Psychiatry 34:1826–1832. https://doi.org/10.1002/gps.5198
Tsuruya K, Yoshida H, Kuroki Y, Nagata M, Mizumasa T, Mitsuiki K, Yoshiura T, Hirakawa M, Kanai H, Hori K, Hirakata H, Kitazono T (2015) Brain atrophy in peritoneal dialysis and CKD stages 3–5: a cross-sectional and longitudinal study. Am J Kidney Dis 65:312–321. https://doi.org/10.1053/j.ajkd.2014.07.011
Solomon MA, van der Plas E, Langbehn KE, Novak M, Schultz JL, Koscik TR, Conrad AL, Brophy PD, Furth SL, Nopoulos PC, Harshman LA (2021) Early pediatric chronic kidney disease is associated with brain volumetric gray matter abnormalities. Pediatr Res 89:526–532. https://doi.org/10.1038/s41390-020-01203-w
van der Plas E, Solomon MA, Hopkins L, Koscik T, Schultz J, Brophy PD, Nopoulos PC, Harshman LA (2022) Global and regional white matter fractional anisotropy in children with chronic kidney disease. J Pediatr 242:166-173.e3. https://doi.org/10.1016/j.jpeds.2021.11.006
Hartung EA, Erus G, Jawad AF, Laney N, Doshi JJ, Hooper SR, Radcliffe J, Davatzikos C, Furth SL (2018) Brain magnetic resonance imaging findings in children and young adults with CKD. Am J Kidney Dis 72:349–359. https://doi.org/10.1053/j.ajkd.2017.11.024
Ismail FY, Fatemi A, Johnston MV (2017) Cerebral plasticity: windows of opportunity in the developing brain. Eur J Paediatr Neurol 21:23–48
Lanphear BP (2015) The impact of toxins on the developing brain. Annu Rev Public Health 36:211–230
Haverman L, van Rossum MA, van Veenendaal M, van den Berg JM, Dolman KM, Swart J, Kuijpers TW, Grootenhuis MA (2013) Effectiveness of a web-based application to monitor health-related quality of life. Pediatrics 131:e533-543. https://doi.org/10.1542/peds.2012-0958
Onderwijsindeling S (2016) Standard educational classification. Den Haag/Heerlen: Centraal Bureau voor de Statistiek [Statistics Netherlands]
Kaldenbach Y (2006) De WISC-III anno 2006. Kind & Adolescent Praktijk 5:100
Field A (2013) Discovering statistics using IBM SPSS statistics: Sage
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393
Hayes AF (2017) Introduction to mediation, moderation, and conditional process analysis: a regression-based approach: Guilford publications
Cohen J (1988) Statistical power analysis for the behavioral sciences. Routledge, New York
O’Lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, Parker D, Whiteley W, Strippoli GF, Palmer SC, Craig JC, Webster AC (2016) Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 67:925–935. https://doi.org/10.1053/j.ajkd.2015.12.028
Toplak ME, West RF, Stanovich KE (2013) Practitioner review: do performance-based measures and ratings of executive function assess the same construct? Child Psychol Psychiatry 54:131–143
Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104. https://doi.org/10.1152/jn.00221.2016
Park HJ, Friston K (2013) Structural and functional brain networks: from connections to cognition. Science 342:1238411. https://doi.org/10.1126/science.1238411
Serrano OK, Bangdiwala AS, Vock DM, Chinnakotla S, Dunn TB, Finger EB, Kandaswamy R, Pruett TL, Najarian JS, Matas AJ, Chavers B (2018) Incidence and magnitude of post-transplant cardiovascular disease after pediatric kidney transplantation: risk factor analysis of 1058 pediatric kidney transplants at the university of Minnesota. Pediatr Transplant 22:e13283. https://doi.org/10.1111/petr.13283
Lande MB, Mendley SR, Matheson MB, Shinnar S, Gerson AC, Samuels JA, Warady BA, Furth SL, Hooper SR (2016) Association of blood pressure variability and neurocognition in children with chronic kidney disease. Pediatr Nephrol 31:2137–2144. https://doi.org/10.1007/s00467-016-3425-2
Haight T, Nick Bryan R, Erus G, Hsieh MK, Davatzikos C, Nasrallah I, D’Esposito M, Jacobs DR Jr, Lewis C, Schreiner P, Sidney S, Meirelles O, Launer LJ (2018) White matter microstructure, white matter lesions, and hypertension: an examination of early surrogate markers of vascular-related brain change in midlife. Neuroimage Clin 18:753–761. https://doi.org/10.1016/j.nicl.2018.02.032
Verbitsky M, Kogon AJ, Matheson M, Hooper SR, Wong CS, Warady BA, Furth SL, Gharavi AG (2017) Genomic disorders and neurocognitive impairment in pediatric CKD. J Am Soc Nephrol 28:2303–2309. https://doi.org/10.1681/asn.2016101108
Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ (2006) Neurobiology of exercise. Obesity (Silver Spring) 14:345–356. https://doi.org/10.1038/oby.2006.46
Meijer A, Pouwels PJW, Smith J, Visscher C, Bosker RJ, Hartman E, Oosterlaan J, Königs M (2021) The relationship between white matter microstructure, cardiovascular fitness, gross motor skills, and neurocognitive functioning in children. J Neurosci Res 99:2201–2215. https://doi.org/10.1002/jnr.24851
Opel N, Martin S, Meinert S, Redlich R, Enneking V, Richter M, Goltermann J, Johnen A, Dannlowski U, Repple J (2019) White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Sci Rep 9:12885. https://doi.org/10.1038/s41598-019-49301-y
Ruotsalainen I, Gorbach T, Perkola J, Renvall V, Syväoja HJ, Tammelin TH, Karvanen J, Parviainen T (2020) Physical activity, aerobic fitness, and brain white matter: their role for executive functions in adolescence. Dev Cogn Neurosci 42:100765. https://doi.org/10.1016/j.dcn.2020.100765
Meijer A, Königs M, Vermeulen GT, Visscher C, Bosker RJ, Hartman E, Oosterlaan J (2020) The effects of physical activity on brain structure and neurophysiological functioning in children: a systematic review and meta-analysis. Dev Cogn Neurosci 45:100828
Kort W, Schittekatte M, Dekker PH, Verhaeghe P, Compaan EL, Bosmans M, Vermeir G (2005) WISC-III NL Wechsler Intelligence Scale for Children. Derde Editie NL. Handleiding en Verantwoording. Amsterdam: Psychologen HTPNIv
Wechsler D (2012) Wechsler Adults Intelligence Scale-Fourth Edition-Nederlandstalige Bewerking. Technische Handleiding. Pearson Assessment and Information BV, Amsterdam
Sattler J (2001) Assessment of children: cognitive applications, 4th edn. Jerome M Sattler Publisher, La Mesa, CA, US
Kingma A, van den Burg W (2008) Drie parallelversies van de 15-woordentest voor kinderen: handleiding & normering: Stichting Kinderneuropsychologie Noord Nederland
Schmand B, Houx P, de Koning I (2012) Normen 15-woordentest [Dutch reference data for the RAVLT]. sectie Neuropsychologie. Nederlands Instituut van Psychologen, Amsterdam
Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System®(D-KEFS®): examiner’s manual: flexibility of thinking, concept formation, problem solving, planning, creativity, impluse control, inhibition: Pearson
Korkman M, Kirk U, Kemp S (2007) NEPSY I: clinical and interpretative manual. Psychological Corporation, San Antonio, TX
Van Overschelde JP, Rawson KA, Dunlosky J (2004) Category norms: an updated and expanded version of the norms. J Mem Lang 50:289–335
Schmand B, Groenink S, Van den Dungen M (2008) Letter fluency: psychometric properties and Dutch normative data. Tijdschr Gerontol Geriatr 39:64–76
Funding
This study was partly funded by the Dutch Kidney Foundation.
Author information
Authors and Affiliations
Contributions
SL, KJO, MSS, FJB, MK, and JWG participated in research design. SL conducted the research and performed data analyses. SL, KJO, MK and JWG were involved in the data analysis plan. MSS, AB, KH, HJ, FJB, and JWG were treating physicians. All authors participated in interpretation of outcomes and participated in the writing of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lijdsman, S., Oostrom, K.J., van Sandwijk, M.S. et al. Risk factors for neurocognitive impairment and the relation with structural brain abnormality in children and young adults with severe chronic kidney disease. Pediatr Nephrol 38, 1957–1969 (2023). https://doi.org/10.1007/s00467-022-05781-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05781-1