Abstract
Background
The advantages of laparoscopy are widely known. Nevertheless, its legitimacy in liver surgery is often questioned because of the uncertain value associated with minimally invasive methods. Our main goal was to compare the outcomes of pure laparoscopic (LLR) and open liver resection (OLR) in patients with hepatocellular carcinoma.
Methods
We searched EMBASE, MEDLINE, Web of Science, and The Cochrane Library databases to find eligible studies. The most recent search was performed on December 1, 2017. Studies were regarded as suitable if they reported morbidity in patients undergoing LLR versus OLR. Extracted data were pooled and subsequently used in a meta-analysis with a random-effects model. Clinical applicability of results was evaluated using predictive intervals. Review was reported following the PRISMA guidelines.
Results
From 2085 articles, forty-three studies (N = 5100 patients) were included in the meta-analysis. Our findings showed that LLR had lower overall morbidity than OLR (15.59% vs. 29.88%, p < 0.001). Moreover, major morbidity was reduced in the LLR group (3.78% vs. 8.69%, p < 0.001). There were no differences between groups in terms of mortality (1.58% vs. 2.96%, p = 0.05) and both 3- and 5-year overall survival (68.97% vs. 68.12%, p = 0.41) and disease-free survival (46.57% vs. 44.84%, p = 0.46).
Conclusions
The meta-analysis showed that LLR is beneficial in terms of overall morbidity and non-procedure-specific complications. That being said, these results are based on non-randomized trials. For these reasons, we are calling for randomization in upcoming studies. Systematic review registration: PROSPERO registration number CRD42018084576.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic liver resection is considered a feasible alternative to the open approach. Minimally invasive techniques have been widely used in the treatment of benign diseases such as hydatid cysts, hepatolithiasis, hemangiomas, focal nodular hyperplasia, and hepatic adenomas [1,2,3]. However, an increasing number of case series of malignant lesions scheduled for the laparoscopic approach have been published in the literature [4]. For instance, in other types of surgery laparoscopic access has been shown to be not inferior to an open approach in terms of oncological outcomes [5,6,7]. Most importantly, there are major well-known advantages related to the minimally invasive approach: less postoperative pain, lower morbidity, faster recovery, and better quality of life [8, 9].
Despite laparoscopy gaining popularity, its clinical utility and complexity in liver surgery, especially extensive liver resection, is still a subject of thorough analysis and discussion [10, 11]. It is often questioned whether reduced complications are enough to outweigh the benefits of open surgery such as the less steep learning curve and potentially shorter operative time. There is, however, evidence supporting non-inferior outcomes, primarily overall survival (OS) and disease-free survival (DFS).
Moreover, operations for hepatocellular carcinoma (HCC) in patients with liver cirrhosis and portal hypertension are considered difficult and may be associated with relatively high morbidity [12]. It has been proved that patients with liver cirrhosis have worse overall outcomes and a higher perioperative complication rate [13, 14].
So far only few meta-analyses comparing laparoscopic and open liver resections for HCC have been performed, and have not taken into consideration variances of the techniques such as pure laparoscopic and hand-assisted. This may create potential bias when drawing conclusions [15,16,17,18]. In addition, these reviews do not cover the many large-scale studies published in recent years.
To our best knowledge, this is the first systematic review and meta-analysis to evaluate exclusively pure laparoscopic liver resection (LLR) compared with open liver resection (OLR) for HCC.
The aim of this study was to evaluate different aspects of LLR, including its safety (morbidity and mortality), difficulty (operative time and blood loss), and clinical utility (long-term survival) in comparison with OLR.
Materials and methods
Search strategy
Our literature search included EMBASE, MEDLINE, Web of Science, and The Cochrane Library databases. The search terms used were “laparoscopy,” “pure laparoscopic,” “minimally invasive,” “liver resection,” “hepatectomy,” “hepatocellular carcinoma,” and their combinations with Boolean “AND” and “OR” operators. There were no date restrictions and only full texts in English were included. Our last search was performed on December 1, 2017. The full search strategy is available in Supplementary File 1. The systematic review was registered and its protocol published in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42018084576.
Study selection
Results of the initial search were screened independently by two teams with three reviewers in each team. Studies containing data comparing morbidity between patients undergoing pure laparoscopic and open liver resection for HCC were considered eligible for inclusion. All studies describing hand-assisted or hybrid resections (without subgroup data on pure laparoscopic resections), national registries, reviews, and animal studies were excluded. Both non-randomized and randomized studies were eligible as long as they matched the inclusion criteria.
Data extraction and outcome measures
Outcomes of this systematic review were overall morbidity, major morbidity, specific complications (bile leak, abscesses, cardiopulmonary), blood loss, surgical site infection rate, conversion rate, operative time, reoperation and readmission rates, R0 resection rate, length of hospital stay, and 3- and 5-year OS and DFS rates. Data on type of study, number of patients enrolled, patients’ age and sex, tumor size, types of surgery, and liver function status (cirrhosis, Child scale) were also extracted. Major morbidity was extracted when stated or—if the Clavien–Dindo scale was used—complications rated as Clavien–Dindo grade 3 and higher were considered major.
Statistical analysis
The analysis was performed using RevMan 5.3 (freeware from The Cochrane Collaboration) and R version 3.4.3 with meta package [19]. Statistical heterogeneity and inconsistency were measured using Cochran’s Q test and I2, respectively. Qualitative outcomes from individual studies were analyzed to assess individual and pooled risk ratios (RR) with pertinent 95% confidence intervals (CI) favoring pure laparoscopic over open liver resection for HCC and by means of the random-effects method. When appropriate, mean and standard deviation (SD) were calculated from medians and interquartile ranges using a method proposed by Hozo et al. [20]. Weighted mean differences with 95% CI are presented for quantitative variables using the inverse variance random-effects method. Statistical significance was observed at the two-tailed 0.05 level for hypothesis and 0.10 level for heterogeneity testing, while unadjusted p values were reported accordingly. To help with clinical interpretation of heterogeneity, we computed prediction intervals (PIs), as suggested by IntHout et al. [21], with the meta R package utilizing the approach of Higgins [22].
Quality assessment
The quality of non-randomized studies was evaluated with the Newcastle–Ottawa Scale (NOS), which consists of three factors: patient selection, comparability of the study groups, and assessment of outcomes. A score ranging from 0 to 9 is assigned to each study, and those that achieve a score of 6 or greater are considered of high quality. The Cochrane risk of bias tool was used to assess the quality of the included randomized controlled trials. We used funnel plots and Egger’s test with meta-regression model to explore possible publication bias [23]. In cases of funnel plot asymmetry, the trim-and-fill method was applied to estimate the cause of asymmetry and correct it [24].
This review was performed strictly following Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [25] and the MOOSE consensus statement [26].
Results
Study identification
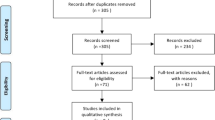
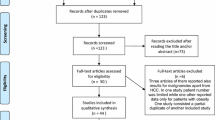
The initial search yielded 3852 articles. After removing 1767 duplicates, 2085 studies were screened by their titles and abstracts for further analysis. Since 1624 did not match the review criteria, 461 full-text articles were screened for eligibility and of these, 418 were later excluded. The PRISMA flowchart and reasons for study exclusion are shown in Fig. 1.
Characteristics of included studies
The characteristics of a total of 5100 patients from 43 studies included in the meta-analysis are specified in Table 1 [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. The only randomized controlled trial was conducted by Jiang et al. [48].
Hospital volume
We estimated the volume of hospitals where studies were performed. Some institutes were noticeably very high-volume centers with almost 3000 cases in 6 years [39], while others performed as few as 60 procedures in 5 years [61].
Study quality
In all included studies, their quality was rated as high (≥ 6 by assessment using the NOS scale), and the risk of bias of the included randomized controlled trial was low according to Cochrane criteria.
Type of surgery
27 studies reported data on types of resections performed, including number of hemihepatectomies, although the reporting style and detail varied between articles.
Liver function
With respect to cirrhosis in patients, 27 studies reported data. Of these, 11 included only patients with cirrhosis. In total, 1065 out of 1257 (84.73%) and 1831 out of 2150 (85.16%) patients with cirrhosis were reported in LLR and OLR groups, respectively. Meanwhile, 33 studies reported data on patients’ Child–Pugh score, with 14 trials analyzing only subjects with Child–Pugh grade A.
Tumor size
35 manuscripts reported on tumor size. There is a noticeable trend of submitting patients with larger lesions to undergo OLR, leading to a potential yet incomputable bias. Pooled estimate analysis showed a significant trend toward smaller tumor sizes in LLR (mean difference − 0.26, 95% CI − 0.42 to − 0.10, p for effect < 0.001). However, the data are highly heterogeneous (I2 = 79%, p < 0.001).
Pringle maneuver
14 articles reported on use of the Pringle maneuver. 163 of 584 (27.91%) LLR patients and 250 of 865 (28.90%) OLR patients underwent this technique during surgery.
Overall morbidity
All studies reported on overall morbidity. The pooled analysis (Fig. 2A) indicates that LLR is connected with reduced overall morbidity (15.59%) compared with OLR (29.88%): RR = 0.53, 95% CI 0.47–0.60, p for overall effect < 0.00001, p for heterogeneity 0.29, I2 = 10%. The funnel plot is asymmetric and Egger’s regression test result significant, both of which indicate potential publication bias. Trim-and-fill analysis was performed and 10 studies that are mirror images of most outlying studies were filled in, [27, 34, 43, 44, 47, 48, 51, 62,63,64] as seen in Fig. 2B. Nevertheless, after this evaluation, the significance of the results remained unchanged, with RR = 0.57, 95% CI 0.49–0.65, and PI = 0.34–0.95.
A Pooled estimates of overall morbidity for pure laparoscopic versus open liver resection for hepatocellular carcinoma. CI confidence interval, df degrees of freedom, MH Mantel–Haenszel. B Funnel plot for results from all studies after trim-and-fill analysis for overall morbidity. White dots represent filled-in studies
Major morbidity and mortality
Major morbidity was reported in 32 studies (n = 4080 patients). Results in only two of them [30, 42] were significantly different, and pooled RRs favor LLR (3.76%) over OLR (8.69%), with RR = 0.48, 95% CI 0.36–0.63, p for effect < 0.00001, p for heterogeneity 0.99, I2 = 0%, and PI virtually equal to CI (Fig. 3A). Thirty-two studies (n = 3657 patients) reported data on mortality. Mortality rate in LLR was 1.58% versus 2.96% in OLR. Pooled analysis showed that mortality was also not significantly different between open and pure laparoscopic groups: RR = 0.64, 95% CI 0.42–1.00, p for effect 0.05, and I2 = 0%. PI was slightly wider than CI, being 0.40–1.04 (Fig. 3B).
Complications
Bile leak
Bile leak rate was reported in 29 studies (n = 3831 patients). There were no significant differences between groups, with rates of 1.70% in the LLR group and 2.33% in the OLR group: RR = 0.77, 95% CI 0.48–1.24, p for effect 0.28, and I2 = 0% (Fig. 4A).
Abscesses
Fifteen trials (n = 2034 patients) reported on abscess occurrence. One out of 780 (0.13%) patients in the LLR group and 14 of 1254 (1.12%) in the OLR group had abscesses. Pooled analysis showed no statistical differences between these groups: RR = 0.40, 95% CI 0.14–1.18 p = 0.10, I2 = 0% (Fig. 4B).
Pulmonary complications
Twenty-eight studies (n = 3343 patients) reported the occurrence of pulmonary complications: 5.01% of patients undergoing LLR and 10.03% in the OLR had this morbidity, its rate being significantly reduced in the LLR group: RR = 0.58, 95% CI 0.44–0.75 (PI 0.43–0.77), p for effect < 0.0001, and I2 = 0% (Fig. 4C).
Blood loss
Data on blood loss were reported in 34 studies (n = 4116 patients). The heterogeneity for this outcome was very high, I2 = 94%. Sensitivity analysis did not find any potential sources of heterogeneity. For this reason, we decided not to pool the results.
Operative time
Operative time was reported in 43 studies (n = 5100 patients). We did not pool the results because of the very high heterogeneity (p < 0.0001, I2 = 91%). Sensitivity analysis did not find specific studies that caused these results.
Length of hospital stay
Length of hospital stay was reported in 42 studies (n = 5032 patients). However, heterogeneity was high (I2 = 85%) and its source was not revealed by sensitivity analysis. Thus, no pooling was performed.
Survival
Three-year OS was reported in 21 studies (n = 2950 patients), while 18 (n = 2467 patients) trials reported 5-year OS. There were no significant variations among the analyzed groups: the LLR group had OS rates of 83.72% and 68.97% in 3 and 5 years, respectively. The OS rate in patients undergoing OLR was 80.82% in 3 years and 68.12% in 5 years. For 3-year OS, RR = 0.86, 95% CI 0.72–1.02, p = 0.08, and I2 = 6% (Fig. 5A). For 5-year OS, RR = 0.94, 95% CI 0.82–1.09, p = 0.41, and I2 = 27% (Fig. 5B).
Nineteen studies (n = 2836 patients) reported on 3-year DFS. Thirteen articles (n = 1829 patients) provided information on 5-year DFS. Pooled analysis showed no differences between groups for either outcome. The 3-year DFS rate was 59.45% in the LLR group and 59.05% in the OLR group, while the 5-year DFS rate was 46.57% for LLR patients and 44.84% for OLR patients. For 3-year DFS, RR = 1.02, 95% CI 0.90–1.15, and p = 0.81. We analyzed sources of moderate heterogeneity (I2 = 47%), and in sensitivity analysis we found two studies that affected the results [42, 64]. After their removal from the meta-analysis, pooling confirmed previous findings, with RR = 1.01 (95% CI 0.93–1.10) and virtually no heterogeneity (I2 = 0%) (Fig. 6A). For 5-year DFS, RR = 0.97, 95% CI 0.90–1.05, p = 0.46, and I2 = 0% (Fig. 6B).
Discussion
Summary of findings
There is growing evidence supporting the feasibility of laparoscopic liver resection for HCC, and its safety is confirmed in our meta-analysis. This review, including over 5000 patients, shows that pure laparoscopy significantly reduces morbidity while at the same time delivering survival comparable with that of open surgery. Because of the very high heterogeneity, it is not possible to definitively assess the differences in blood loss and operative time. Although the quality of all included studies was assessed using standardized tools as high, all but one are non-randomized, which may introduce selection bias. Moreover, there were differences in tumor size and in the use of the Pringle maneuver between LLR and OLR groups, which may cause serious bias and troublesome interpretation of results.
In addition, we did not include three studies because of the language limitations. However, based on abstract screening, number of cases in them was relatively small (less than 50 cases). Therefore, it is very unlikely that their inclusion would alter the final results.
We realize that our review is not the first to be conducted on this topic. However, previously published meta-analyses on liver resections for HCC either did not take the type of laparoscopic technique into consideration [16,17,18] or performed a subgroup analysis that mistakenly assigned trials with hybrid resections to the LRR group as in Sotiropoulos et al. [15]. This might have introduced a major methodological bias to previous studies. In addition, more recent large-scale trials are not included in previous reviews. Moreover, several meta-analyses, including recently published one by Goh et al., were limited to cirrhotic patients, which does not allow to draw conclusions with wide clinical applicability of laparoscopy [70].
These facts prompted us to revisit this topic and follow strict methodological and surgical criteria to obtain the best available evidence. Additionally, we used PIs to interpret whether the results would be applicable in different clinical settings.
When defining the aim of our study, we strived to tackle the issue on different levels in terms of LLR safety (by analyzing morbidity, mortality, and specific complications), difficulty (operative time and blood loss), and its long-term results (OS and DFS).
LLR safety
Overall morbidity is crucial in our review for assessment of the method’s safety. Pooled analysis confirmed the benefits of laparoscopy with low heterogeneity and its demonstrable effect in different settings. It is worth noting that only one study, by Hu et al. [61], reported higher overall morbidity in LLR. Also, common general (i.e., pulmonary) complications were less likely to occur in the LLR patients, while procedure-associated complications (bile leak, abscesses) did not differ compared with OLR.
Nevertheless, results varied between studies: some showed an overall complication rate as high as 20.31% for LLR [42] while others reported no morbidity at all among 50 patients undergoing LLR [48]. Such discrepancies can be explained to some extent by hospital volume or surgeon experience, as discussed further in the limitations of our review below, but it seems that the definitions and reporting of specific complications are not standardized, which may result in significant discrepancies between included studies and eventual bias.
Pooled analysis also showed no differences in mortality (1.58% in LLR vs. 2.96% in OLR; RR = 0.64; 95% CI 0.42–1.00), which generally is a relatively rare complication in liver resections for HCC. Only one study by Xiang et al. [42] had a higher mortality rate in the LLR group, but it is important to note that this was based on one death in both groups: 1 out of 128 in LLR and 1 out of 208 in OLR.
Difficulty of LLR and OLR
Laparoscopy in surgery is sometimes disparaged as being more complex, supposedly because of the steep learning curve, longer operation times, and greater blood loss [71]. However, it has been shown that more experienced surgeons have, in fact, shorter median operative times as well as reduced blood loss and conversion rates in liver surgery [72]. Many parameters, such as hospital volume, are difficult to compare, which directly affects the experience and subsequent intraoperative results. Some studies point out that an increase in operative time may be a result of the learning curve and should improve in the future [69]. Others, however, claim to have lowered it, as evidenced by a reduced conversion rate [36, 49]. This learning curve effect is nearly impossible to include in an analysis. These study limitations may be one reason why the legitimacy of LLR is often challenged in liver surgery. In our meta-analysis, we decided not to perform a pooled analysis of operative time, blood loss, and length of stay for reasons of significant heterogeneity. Even if pooling was possible, there is a potential bias because of highly variable operative techniques between centers. Most studies did not thoroughly describe the type of surgical devices and techniques of parenchyma transection, which influences the total blood loss. Another difference is the rate of use of the Pringle maneuver.
Usually laparoscopy is also associated with extended duration of surgery, but there is a possibility of patient selection bias reflecting surgeons’ preference to submit more complex cases to OLR. However, recent analyses showed that in liver resections a trend toward shorter operative time in laparoscopy may in fact be non-significant [73, 74]. Types of surgical instruments used in LLR may also affect the operative time [75].
The very high heterogeneity of these results (I2 = 94% for blood loss, I2 = 91% for operative time, and I2 = 85% for length of stay) does not allow us to draw definitive conclusions, and it would seem that non-randomized trials may not be able to resolve this issue.
Long-term results
The meta-analysis confirmed previous findings [17, 18] that LLR does not differ from OLR in terms of OS and DFS. This has to be juxtaposed with the clear benefits of LLR safety as well as its vague yet potentially greater difficulty. Our meta-analysis of more than 5000 cases points out the weakness of non-randomized trials that do not allow for unequivocal conclusions. All studies but one, by Jiang and Cao [48], presented non-randomized groups. This, in our opinion, is a massive drawback that must be taken into account when discussing the data. We interpret our results as a plea for well-designed multicenter trials analyzing the type of surgery, complexity of the procedure, surgeon’s experience, and hospital volume.
Due to inconsistent reporting in included manuscripts, we did not analyze recurrence-free survival separately from DFS. Although a few publications did indeed evaluate recurrences in LLR versus OLR, mean follow-up time varies significantly between studies, making it impossible to pool results without bias.
A very important bias results from patient allocation. There are indisputable differences between LLR and OLR patients regarding the tumor size, cirrhosis, or, intraoperatively, use of the Pringle maneuver. This limitation can only be overcome by randomized controlled trials. Moreover, center volumes highly differ, as does surgeons’ experience. The latter was not reported in our included studies despite it being an extremely important factor in complex liver surgeries. In addition, use of the Pringle maneuver reported in the studies varies and resulted in very high heterogeneity. Finally, we did not analyze the potential impact of surgical technique and its differences between studies. Neither were perioperative care protocols considered, despite their application having been shown to be beneficial in many surgical disciplines [76, 77].
Conclusions
This systematic review with a meta-analysis, thus far the most comprehensive analysis comparing pure LLR with OLR for HCC, reveals major flaws in the available literature. The results indicate that LLR is safe in different clinical settings as it may be associated with reduced overall morbidity and non-procedure-specific complications, and no negative influence on mortality as well as OS and DFS. However, these results are based on non-randomized trials comparing heterogeneous groups of patients, thus introducing confounding variables from the outset.
In our opinion, therefore, there is no need for further non-randomized trials proving the feasibility and safety of LLR. This is a plea for large, multicenter, well-designed randomized controlled trials that can overcome the weaknesses of the available evidence.
References
Yagmur Y, Akbulut S, Gumus S, Babur M, Can MA (2016) Laparoscopic management of hydatid cyst of the liver. S Afr J Surg 54:14–17
Li H, Zheng J, Cai J-Y, Li S-H, Zhang J-B, Wang X-M, Chen G-H, Yang Y, Wang G-S (2017) Laparoscopic VS open hepatectomy for hepatolithiasis: an updated systematic review and meta-analysis. World J Gastroenterol 23:7791–7806. https://doi.org/10.3748/wjg.v23.i43.7791
van Rosmalen BV, Bieze M, Besselink MGH, Tanis P, Verheij J, Phoa SSKS, Busch O, van Gulik TM (2016) Long-term outcomes of resection in patients with symptomatic benign liver tumours. HPB (Oxford) 18:908–914. https://doi.org/10.1016/j.hpb.2016.07.013
Coelho FF, Kruger JAP, Fonseca GM, Araujo RLC, Jeismann VB, Perini MV, Lupinacci RM, Cecconello I, Herman P (2016) Laparoscopic liver resection: experience based guidelines. World J Gastrointest Surg 8:5–26. https://doi.org/10.4240/wjgs.v8.i1.5
Leon P, Iovino MG, Giudici F, Sciuto A, de Manzini N, Cuccurullo D, Corcione F (2017) Oncologic outcomes following laparoscopic colon cancer resection for T4 lesions: a case-control analysis of 7-years’ experience. Surg Endosc. https://doi.org/10.1007/s00464-017-5784-6
Pędziwiatr M, Małczak P, Mizera M, Witowski J, Torbicz G, Major P, Pisarska M, Wysocki M, Budzyński A (2017) There is no difference in outcome between laparoscopic and open surgery for rectal cancer: a systematic review and meta-analysis on short- and long-term oncologic outcomes. Tech Coloproctol. https://doi.org/10.1007/s10151-017-1662-4
Pędziwiatr M, Małczak P, Pisarska M, Major P, Wysocki M, Stefura T, Budzyński A (2017) Minimally invasive versus open pancreatoduodenectomy—systematic review and meta-analysis. Langenbeck’s Arch Surg 402:841–851. https://doi.org/10.1007/s00423-017-1583-8
Lorenzon L, La Torre M, Ziparo V, Montebelli F, Mercantini P, Balducci G, Ferri M (2014) Evidence based medicine and surgical approaches for colon cancer: evidences, benefits and limitations of the laparoscopic vs open resection. World J Gastroenterol 20:3680–3692. https://doi.org/10.3748/wjg.v20.i13.3680
Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath F-A (2015) Laparoscopic colorectal surgery confers lower mortality in the elderly: a systematic review and meta-analysis of 66,483 patients. Surg Endosc 29:322–333. https://doi.org/10.1007/s00464-014-3672-x
Iwata T, Murotani K, Komatsu S, Mishima H, Arikawa T (2018) Surgical outcome of laparoscopic hepatic resection for hepatocellular carcinoma: a matched case-control study with propensity score matching. J Minim Access Surg 14:277–284. https://doi.org/10.4103/jmas.JMAS_116_17
Xu H-W, Li H-Y, Liu F, Wei Y-G, Li B (2017) Laparoscopic versus open liver resection for lesions adjacent to major vessels: a propensity score matched analysis. J Laparoendosc Adv Surg Tech A 27:1002–1008. https://doi.org/10.1089/lap.2017.0326
Hackl C, Schlitt HJ, Renner P, Lang SA (2016) Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol 22:2725–2735. https://doi.org/10.3748/wjg.v22.i9.2725
Araujo L, Dombrovskiy V, Kamran W, Lemaire A, Chiricolo A, Lee LY, Lemaire A (2017) The effect of preoperative liver dysfunction on cardiac surgery outcomes. J Cardiothorac Surg 12:73. https://doi.org/10.1186/s13019-017-0636-y
Han EC, Ryoo S-B, Park JW, Yi JW, Oh H-K, Choe EK, Ha H-K, Park BK, Moon SH, Jeong S-Y, Park KJ (2017) Oncologic and surgical outcomes in colorectal cancer patients with liver cirrhosis: a propensity-matched study. PLoS ONE 12:e0178920
Sotiropoulos GC, Prodromidou A, Kostakis ID, Machairas N (2017) Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg 69:291–311. https://doi.org/10.1007/s13304-017-0421-4
Xiong J-J, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu X-B, Hu W-M (2012) Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 18:6657–6668. https://doi.org/10.3748/wjg.v18.i45.6657
Yin Z, Fan X, Ye H, Yin D, Wang J (2013) Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 20:1203–1215. https://doi.org/10.1245/s10434-012-2705-8
Parks KR, Kuo Y-H, Davis JM, O’ Brien B, Hagopian EJ (2014) Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB 16:109–118. https://doi.org/10.1111/hpb.12117
Schwarzer G, Carpenter JR, Rücker G (2015) Meta-analysis with R. Springer, New York
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6:e010247. https://doi.org/10.1136/bmjopen-2015-010247
Higgins JPT, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A 172:137–159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283:2008–2012
Amato B, Aprea G, De Rosa D, Milone M, di Domenico L, Amato M, Compagna R, Santoro M, Johnson LB, Sanguinetti A, Polistena A, Avenia N (2017) Laparoscopic hepatectomy for HCC in elderly patients: risks and feasibility. Aging Clin Exp Res 29:179–183. https://doi.org/10.1007/s40520-016-0675-6
Chen J, Li H, Liu F, Li B, Wei Y (2017) Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 96:e6460. https://doi.org/10.1097/MD.0000000000006460
Guro H, Cho JY, Han H-S, Yoon Y-S, Choi Y, Jang JS, Kwon SU, Kim S, Choi JK (2017) Laparoscopic liver resection of hepatocellular carcinoma located in segments 7 or 8. Surg Endosc. https://doi.org/10.1007/s00464-017-5756-x
Li W, Zhou X, Huang Z, Zhang K, Luo X, Zhong J, Chen Y (2017) Short-term and long-term outcomes of laparoscopic hepatectomy, microwave ablation, and open hepatectomy for small hepatocellular carcinoma: a 5-year experience in a single center. Hepatol Res 47:650–657. https://doi.org/10.1111/hepr.12785
Tarantino G, Magistri P, Serra V, Berardi G, Assirati G, Ballarin R, Di Benedetto F (2017) Laparoscopic liver resection of right posterior segments for hepatocellular carcinoma on cirrhosis. J Laparoendosc Adv Surg Tech 27:559–563. https://doi.org/10.1089/lap.2016.0506
Xu H, Liu F, Li H, Wei Y, Li B (2017) Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. https://doi.org/10.1007/s00464-017-5727-2
Xu X, Chen J, Wang F, Ni Q, Naimat U, Chen Z (2017) Recurrence of hepatocellular carcinoma after laparoscopic hepatectomy: risk factors and treatment strategies. J Laparoendosc Adv Surg Tech 27:676–684. https://doi.org/10.1089/lap.2016.0541
Yoon Y, Kim ÃK, Kang ÃS, Kim W, Shin M, Lee ÃS, Jung ÃD, Park ÃG (2017) Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 265:856–863. https://doi.org/10.1097/SLA.0000000000002072
Ahn S, Cho A, Kim EK, Paik KY (2016) Favorable long-term oncologic outcomes of hepatocellular carcinoma following laparoscopic liver resection. J Laparoendosc Adv Surg Tech 26:447–452. https://doi.org/10.1089/lap.2015.0534
Cheung TT, Dai WC, Tsang SHY, Chan ACY, Chok KSH, Chan SC, Lo CM (2016) Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis. Ann Surg 264:612–620. https://doi.org/10.1097/SLA.0000000000001848
Harada N, Maeda T, Yoshizumi T, Ikeda T, Kayashima H, Ikegami T, Harimoto N, Takaki S, Maehara Y (2016) Laparoscopic liver resection is a feasible treatment for patients with hepatocellular carcinoma and portal hypertension. 3498:3489–3497
Jiang X, Liu L, Zhang Q, Jiang Y, Huang J, Zhous H, Zeng L (2016) Laparoscopic versus open hepatectomy for hepatocellular carcinoma: long-term outcomes. JBUON 21:135–141
Lai C, Jin R-A, Liang X, Cai X-J (2016) Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 17:236–246. https://doi.org/10.1631/jzus.B1500322
Sotiropoulos GC, Machairas N, Stamopoulos P, Kostakis ID, Dimitroulis D, Mantas D, Kouraklis G (2016) Laparoscopic versus open liver resection for hepatocellular carcinoma: initial experience in Greece. Ann Gastroenterol 29:521–529. https://doi.org/10.20524/aog.2016.0067
Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, Romito R, Mariani L, Mazzaferro V (2016) Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 103:871–880. https://doi.org/10.1002/bjs.10137
Xiang L, Li J, Chen J, Wang X, Guo P, Fan Y, Zheng S (2016) Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg 103:1895–1901. https://doi.org/10.1002/bjs.10294
Zhang Y, Chen X-M, Sun D-L (2016) Short-term outcomes of laparoscopic versus open right hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 26:e157–e160. https://doi.org/10.1097/SLE.0000000000000355
Zhang Y, Huang J, Chen X-M, Sun D-L (2016) A comparison of laparoscopic versus open left hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 26:146–149. https://doi.org/10.1097/SLE.0000000000000247
Cho JY, Han HS, Yoon YS, Choi Y, Lee W (2015) Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surg (United States) 158:135–141. https://doi.org/10.1016/j.surg.2015.02.007
Han H-S, Shehta A, Ahn S, Yoon Y-S, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650. https://doi.org/10.1016/j.jhep.2015.04.005
Harimoto N, Ikeda T, Takeishi K, Itoh S, Yamashita YI, Ikegami T, Yoshizumi T, Kawanaka H, Shirabe K, Maehara Y (2015) Outcomes after laparoscopic hepatectomy in the semi-prone position for hepatocellular carcinoma located in segment 6, 7, or 8. Anticancer Res 35:4167–4170
Jiang H, Cao J (2015) Impact of laparoscopic versus open hepatectomy on perioperative clinical outcomes of patients with primary hepatic carcinoma Hai-tao. Chin Med Sci J 30:80–83
Lee JJ, Conneely JB, Smoot RL, Gallinger S, Greig PD, Moulton CA, Wei A, McGilvray I, Cleary SP (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: a 2-to-1 matched pair analysis. Hpb 17:304–310. https://doi.org/10.1111/hpb.12342
Luo L, Zou H, Yao Y, Huang X (2015) Laparoscopic versus open hepatectomy for hepatocellular carcinoma: short- and long-term outcomes comparison. Int J Clin Exp Med 8:18772–18778
Tanaka S, Takemura S, Shinkawa H, Nishioka T, Hamano G, Kinoshita M, Ito T, Kubo S (2015) Outcomes of pure laparoscopic versus open hepatic resection for hepatocellular carcinoma in cirrhotic patients: a case-control study with propensity score matching. Eur Surg Res 55:291–301. https://doi.org/10.1159/000439274
Xiao L, Xiang L, Li J, Chen J, Fan Y, Zheng S (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc 29:2994–3001. https://doi.org/10.1007/s00464-015-4214-x
Yoon SY, Kim KH, Jung DH, Yu A, Lee SG (2015) Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 29:2628–2634. https://doi.org/10.1007/s00464-014-3980-1
Ahn KS, Kang KJ, Kim YH, Kim T-S, Lim TJ (2014) A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 24:872–877. https://doi.org/10.1089/lap.2014.0273
Kim S-J, Jung H-K, Lee D-S, Yun S-S, Kim H-J (2014) The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res 86:61. https://doi.org/10.4174/astr.2014.86.2.61
Memeo R, De’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, Azoulay D (2014) Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg 38:2919–2926. https://doi.org/10.1007/s00268-014-2659-z
Siniscalchi A, Ercolani G, Tarozzi G, Gamberini L, Cipolat L, Pinna AD, Faenza S (2014) Laparoscopic versus open liver resection: differences in intraoperative and early postoperative outcome among cirrhotic patients with hepatocellular carcinoma—a retrospective observational study. HPB Surg 2014:1–7. https://doi.org/10.1155/2014/871251
Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y (2014) Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg 219:1117–1123. https://doi.org/10.1016/j.jamcollsurg.2014.09.003
Ai J, Li J, Chen J, Bie P, Wang S, Zheng S-G (2013) Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5–10 cm. PLoS ONE 8:e72328. https://doi.org/10.1371/journal.pone.0072328
Kobayashi S, Nagano H, Marubashi S, Kawamoto K, Wada H, Eguchi H, Tanemura M, Umeshita K, Doki Y, Mori M (2013) Hepatectomy based on the tumor hemodynamics for hepatocellular carcinoma: a comparison among the hybrid and pure laparoscopic procedures and open surgery. Surg Endosc Other Interv Tech 27:610–617. https://doi.org/10.1007/s00464-012-2499-6
Hu BS, Chen K, Tan HM, Ding XM, Tan JW (2011) Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol 17:4725–4728. https://doi.org/10.3748/wjg.v17.i42.4725
Ker CG, Chen JS, Kuo KK, Chuang SC, Wang SJ, Chang WC, Lee KT, Chen HY, Juan CC (2011) Liver surgery for hepatocellular carcinoma: laparoscopic versus open approach. Int J Hepatol 2011:596792. https://doi.org/10.4061/2011/596792
Kim HH, Park EK, Seoung JS, Hur YH, Koh YS, Kim JC, Cho CK, Kim HJ (2011) Liver resection for hepatocellular carcinoma: Case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc 80:412–419. https://doi.org/10.4174/jkss.2011.80.6.412
Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P (2011) Long-term results: of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 35:2268–2274. https://doi.org/10.1007/s00268-011-1212-6
Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR (2011) Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc Other Interv Tech 25:3668–3677. https://doi.org/10.1007/s00464-011-1775-1
Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102:82–86. https://doi.org/10.1002/jso.21541
Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I (2010) Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc Other Interv Tech 24:1170–1176. https://doi.org/10.1007/s00464-009-0745-3
Belli G, Fantini C, D’Agostino A, Cioffi L, Langella S, Russolillo N, Belli A (2007) Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc Other Interv Tech 21:2004–2011. https://doi.org/10.1007/s00464-007-9503-6
Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL (2003) Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 138:763–769. https://doi.org/10.1001/archsurg.138.7.763
Goh EL, Chidambaram S, Ma S (2018) Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a meta-analysis of the long-term survival outcomes. Int J Surg 50:35–42. https://doi.org/10.1016/j.ijsu.2017.12.021
Pascual M, Salvans S, Pera M (2016) Laparoscopic colorectal surgery: current status and implementation of the latest technological innovations. World J Gastroenterol 22:704–717. https://doi.org/10.3748/wjg.v22.i2.704
Kluger MD, Vigano L, Barroso R, Cherqui D (2013) The learning curve in laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 20:131–136. https://doi.org/10.1007/s00534-012-0571-1
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777. https://doi.org/10.1097/SLA.0000000000001413
Croome KP, Yamashita MH (2010) Laparoscopic vs open hepatic resection for benign and malignant tumors: an updated meta-analysis. Arch Surg 145:1109–1118. https://doi.org/10.1001/archsurg.2010.227
Nguyen K, Marsh J, Tsung A, JL S, Gamblin T, DA G (2011) Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 146:348–356
Pisarska M, Pędziwiatr M, Małczak P, Major P, Ochenduszko S, Zub-Pokrowiecka A, Kulawik J, Budzyński A (2016) Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg 36:377–382. https://doi.org/10.1016/j.ijsu.2016.11.088
Pedziwiatr M, Pisarska M, Kisielewski M, Major P, Mydlowska A, Rubinkiewicz M, Winiarski M, Budzynski A (2016) ERAS protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med Oncol 33:56. https://doi.org/10.1007/s12032-016-0772-6
Acknowledgements
This study received no external financial support. We thank Hugh McGonigle, from Edanz Group (http://www.edanzediting.com/ac), for editing a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jan Witowski, Mateusz Rubinkiewicz, Magdalena Mizera, Michał Wysocki, Natalia Gajewska, Mateusz Sitkowski, Piotr Małczak, Piotr Major, Andrzej Budzyński, and Michał Pędziwiatr have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Witowski, J., Rubinkiewicz, M., Mizera, M. et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc 33, 1491–1507 (2019). https://doi.org/10.1007/s00464-018-6431-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6431-6