Abstract
Background
Liver surgery, especially for cirrhotic patients, is one of the last areas of resistance to progress in laparoscopic surgery. This study compares the postoperative results and the 2-year patient outcomes between laparoscopic and open resection for hepatocellular carcinoma in patients with histologically proven cirrhosis.
Methods
From May 2000 to October 2004, 23 consecutive cirrhotic patients who underwent laparoscopic hepatectomy (LH) for HCC were compared in a retrospective analysis with a historic group of 23 patients who underwent open hepatectomy (OH). The two groups were well matched for age, gender, American Society of Anesthesiology (ASA) class, tumor location and size, type of liver resection, and severity of cirrhosis. The selection criteria for both groups specified a small (size < 5 cm), exophytic, or subcapsular tumor located in the left or peripheral right segments of the liver (II–VI segments, Couinaud); a well-compensated cirrhosis (Child-Pugh A); and an ASA score lower than 3. In the LH group, 15 subsegmentectomies, 3 segmentectomies, and 5 left lateral sectionectomies were performed, as compared with 12 subsegmentectomies, 5 segmentectomies, and 6 left lateral sectionectomies in the OH group.
Results
One patient in the LH group (4.3%) underwent conversion to laparotomy for inadequate exposition. The mean operative time was statistically longer for the LH group (LH, 148 min; OH, 125 min; p = 0.016), whereas blood transfusions (LH, 0%; OH, 17.3%; p = 0.036), Pringle maneuver (LH, 0%; OH, 21.73%; p = 0.017), mean hospital stay (LH, 8.3 days; OH, 12 days; p = 0.047), and postoperative complications (LH, 13%; OH, 47.8%; p = 0.010) were significantly greater in OH group. There was no statistically significant difference in mortality and 2-year survival rates between the two groups.
Conclusion
This study shows that LH for HCC in properly selected cirrhotic patients results in fewer early postoperative complications and a shorter hospital stay than the traditional OH. The 2-year survival rate was the same for LH and OH.
Similar content being viewed by others
Liver resection is a worldwide well-accepted treatment for hepatocellular carcinoma (HCC) in patients with cirrhosis, resulting in a high curative rate, a morbidity rate of 10% to 40%, and a mortality rate less than 10% when performed in specialized centers [1, 2]. Since the first laparoscopic liver wedge resection was reported in 1992 [3], limited series of laparoscopic hepatic procedures showing their feasibility, safety, and adequacy have been published in increasing numbers [4–15].
In contrast, only a few reports exist regarding the usefulness, morbidity, and mortality of the mini-invasive approach to HCC for cirrhotic patients [13, 16, 17]. The great potential benefits from using a mini-invasive approach for cirrhotic patients has induced us since 2000 to perform laparoscopic surgery for hepatic lesions, especially HCCs, in these patients [12].
To the best of our knowledge, comparative data, either retrospective or prospective, about laparoscopic treatment of hepatocellular carcinoma for patients with chronic liver disease or cirrhosis are limited to two reports from an eastern country [16, 17] and one report from a western country [13]. All other reports concerned laparoscopic versus open hepatic resection independent of the nature of the lesion (benign or malignant) [9–11, 14].
In this report, we address the indications, evaluate the degree of invasiveness, and assess the oncologic efficacy of laparoscopic hepatectomy for HCC in patients with cirrhosis. We compare the data with that for a historic group of patients who underwent a conventional open liver resection. In this comparison, we analyze the advantages and disadvantages of the mini-invasive approach. We also discuss the importance of the mini-invasive procedure from the viewpoints of both short- and middle- term outcomes.
Materials and methods
From May 2000 to October 2004, 59 laparoscopic hepatic resections for benign and malignant liver diseases were performed in the Department of General and Hepato-Pancreato-Biliary Surgery at S. M. Loreto Nuovo Hospital, Naples, Italy. Of the 59 resections, 23 (39%) were performed for HCC in cirrhotic patients. During the same period, we performed 83 open liver resections for HCC.
The selection criteria for a laparoscopic approach included well-compensated chronic liver disease (Child-Pugh class A) without signs of severe portal hypertension (esophageal varices ≤ F2); exophytic or subcapsular tumors located in the left (II-III-IVb) or peripheral right (V–VI) segments; a maximum lesion size of 4 to 5 cm, and limited resection (<3 segments). Patients with complicated cirrhosis (Child-Pugh class B–C) or an American Society of Anesthesiology (ASA) classification greater than 2 were excluded from the study.
The preoperative workup consisted of a specified protocol including blood examinations, abdominal ultrasound, angiocomputed tomography (CT) scan, esophagogastroduodenoscopy, and spirometry. In selected cases, we performed angiomagnetic resonance imaging (MRI) or, more recently, contrast-enhanced harmonic sonography. Evaluation of hepatic function was done using the Child-Pugh classification of liver dysfunction.
The 23 patients who underwent a mini-invasive liver resection formed the laparoscopic hepatectomy (LH) group. This group included 13 men and 10 women with a mean age of 59.5 ± 6.8 years (range, 49–72 years).
All the patients presented with HCC (some completely exophytic) [18] complicating a chronic liver disease related to hepatitis C virus infection. All the patients in our group had a well-compensated (Child-Pugh class A) and histologically confirmed cirrhosis classified according to the Ishak score for fibrosis (F5 for 3 patients; F6 for 20 patients).
The average size of the lesions was 3.1 ± 0.7 cm (range, 1–3.9 cm). The overall distribution of the lesions according to Couinaud’s classification was as follows: 10 in hepatic segments V–VI, 3 in segment IV, and 10 in the anatomic left lateral lobe (segments II–III). In seven patients, esophagogastroduodenoscopy provided evidence of initial esophageal varices (grade F1). None of the patients had undergone previous abdominal operations. There were 18 ASA 1 cases and 5 ASA 2 cases.
Liver resections were defined according to Brisbane 2000 classification [19] using the following terminology: left lateral sectionectomy (for bisegmentectomy of segments II–III), segmentectomy (for resection of one segment), and subsegmentectomy (for resection of less than one segment). We performed 15 subsegmentectomies, 5 left lateral sectionectomies, and 3 segmentectomies (segment VI in two patients and segment III in one patient).
The clinical data for the 23 patients undergoing laparoscopic hepatectomy were compared with the data for 23 conventional open liver resections. The open hepatectomy (OH) group was selected from patients who underwent surgery from 1995 to 1999 by the same surgeon. The patients were well matched for age, gender, ASA class, tumor location and size, type of liver resection, and severity of liver disease (Child-Pugh, esophageal varices).
The mean age of the 23 patients (14 men and 9 women) in the OH group was 62.4 ± 7.7 years (range, 51–74 years). The average size of the lesions was similar to that found in the LH group (3.24 ± 0.70 cm). The tumor location involved segments V–VI in 12 cases, segment IV in 4 cases, and segments II–III in 7 cases. There were 16 ASA I cases, and 7 ASA II cases. In terms of Child-Pugh class, 14 patients were A5, and 9 patients were A6. In the OH group, 12 subsegmentectomies, 5 segmentectomies, and 6 left lateral sectionectomies were performed.
All the patients were followed with a standard oncologic protocol of surveillance that included a CT scan or, since 2000, a multislice CT scan followed by liver function testing, serum α-fetoprotein level, and ultrasonography 3 months after resection. The serum α-fetoprotein level and abdominal ultrasound were repeated every 3 months, and the CT scan or multislice CT scan were repeated every 6 months.
Surgical procedure
All the operations were performed with the patient under general anaesthesia. The detailed laparoscopic surgical technique that we routinely use in our department has been described in previous reports [12, 20]. Briefly, the patients were placed supine in the “French” position with the primary surgeon positioned between the spread legs. In the case of lesions sited in right lateral segments, the patients were placed in a moderate left lateral decubitus position.
Using an open technique, continuous carbon dioxide (CO2) pneumoperitoneum was induced at a pressure lower than 12 mmHg to prevent the risk of gas embolism. We usually used four (rarely five) 5- to 12-mm trocars and a 30° laparoscope. The trocars were positioned according to the location of the liver lesion, usually along a semicircular line with the concavity facing the right subcostal margin. A standard diagnostic and staging laparoscopy was performed. The liver was evaluated with the aid of a laparoscopic ultrasound probe to confirm the extension of the tumor, the number of lesions, and their position in relation to the main hepatic structures. Neither mobilization of the liver nor round ligament transection was performed. Next, a tape was placed around the porta hepatis and passed through a 16-Fr rubber drain for use as a tourniquet to enable performance of a Pringle maneuver if necessary. The area to be resected was marked by electrocautery.
The parenchymal transection was performed using the harmonic scalpel (Ultracision; Ethicon Endosurgery, Cincinnati, OH, USA) or a new Ligasure device (Ligasure Five; Valleylab, Boulder, CO, USA). Bipolar electrocoagulation also was available for minor bleeding. Intraparenchymal control of the major vessels was achieved with clips, whereas biliary and vascular radicle division was obtained with clips or stapling devices. In case of a left lateral sectionectomy, we performed transection of the liver parenchyma together with sectioning of the vascular pedicle for segments II–III and of the left hepatic vein using consecutive linear staplers (vascular cartridge).
The resected, undivided specimen was placed in a plastic bag and removed through the slightly enlarged periumbilical incision or a horizontal minilaparotomy in the suprapubic region, thus enabling histologic review. The Argon Beam coagulator (Force FX; Valleylab, Boulder, CO, USA) was sometimes applied on the raw surface of the liver to control blood oozing from the stump while abdominal pressure (<15 mmHg) was monitored to prevent the risk of gas embolism. All resection bed surfaces were treated with a biologic fibrin sealant (Tissucol; Baxter, Vienna, Austria) or with a new hemostatic gel (Floseal; Baxter) to minimize the risk of biliary leak and to ensure hemostasis.
The open hepatectomies were performed via a right subcostal incision, extended in a few cases to the left. A complete liver mobilization, obtained by section of all the suspensory ligaments, was considered indispensable to a complete intraoperative ultrasound examination. The hepatic pedicle was always isolated to enable performance of the Pringle maneuver when needed.
Parenchymal transection was achieved with crushing forceps. Bipolar electrocoagulation was used for minor bleeding. Intraparenchymal control of the major vessels was obtained with clips or nonabsorbable sutures.
Statistical analysis
The surgical procedure, postoperative course, and 2-year follow-up evaluation were studied. The following criteria were evaluated and compared between the two groups of patients: surgical time, need for and duration of the Pringle maneuver (when used), blood loss, transfusion rate, pathologic margins, postoperative complications, perioperative mortality, hospital stay, 2-year survival rate, and local recurrences. We studied the 2-year outcome, even if some patients had a longer follow-up period, so all patients had at least 24 months of follow-up evaluation.
The data are expressed as means ± standard deviation. Comparison of quantitative variables was performed using the chi-square (χ2) test and Fisher’s exact test. The Student’s t-test was used to compare continuous variables. Patient survival was calculated by the product limit method of Kaplan and Meier, and the differences in survival between the groups were compared using the log-rank test. A p value less than 0.05 was considered statistically significant.
Results
The two groups were well matched in terms of demographic data, tumor features, severity of cirrhosis, ASA status, and operative procedures (Table 1).
In the LH group, the laparoscopic procedure was successfully completed for 22 patients. Conversion to laparotomy was needed for one patient (4.3%) with an HCC preoperatively localized in segment VI but intraoperatively found to be posterior in segment VII. The exposure was considered insufficient for a safe and oncologic adequate procedure.
The results data are reported in Table 2. No intraoperative complications occurred in the entire study cohort. The mean operative time was significantly longer in the LH group by an average of 20 min (p = 0.01) despite the fact that mean operative time decreased to less than 120 min in the last 10 laparoscopic procedures.
In the LH group, the Pringle maneuver was prepared for 13 patients (56.5%), but it was never used. For 10 patients, it was not absolutely prepared because of large collateral veins or adhesions. In OH group, the Pringle maneuver was prepared in all cases, but only for five patients (21.7%; p = 0.017) was intermittent portal triad clamping (15 min clamping and 5 min release periods) used for a mean period of 30 ± 15 min (range, 15–45 min) to avoid an intraoperative hemorrhage.
The blood loss was similar in the two groups, but there were no intraoperative or perioperative blood transfusions in the LH group. In contrast, four patients (17.3%) in the OH group were infused with 2 units of packed red blood cells (p = 0.036).
Surgical margins were examined in all the histological reports. In the LH group, 2 early patients (8.6%) of the 23 showed a surgical margin less than 1 cm (respectively, 0.5 and 0.7 cm), but in these cases, the tumor was encapsulated, and the capsule had not been invaded at histology. In the OH group, all the patients had resection margins greater than 1 cm.
The postoperative medical treatment was similar for the two groups including intravenous electrolyte and balanced fluid solutions. Oral intake of fluid started on postoperative day 2. The patients usually were given a low sodium diet. Intravenous furosemide was given at early signs of fluid retention.
There was one death (4.3%) in the LH group. This patient died on postoperative day 3 of acute respiratory distress syndrome. There were no deaths in the OH group, but this difference was not statistically significant.
The morbidity rate was 13% (3 patients) in the LH group and 47.8% (11 patients) in the OH group (p = 0.01). More precisely, in the laparoscopic group, three patients experienced transient postoperative ascitis (defined as clinically detectable or as abdominal drainage output, when present, of 500 ml or more per day), which resolved successfully with conservative treatment (diuretics). In the open group, 11 patients experienced 17 complications. More details, according to Dindo classification [21], are reported in Table 3. There was no instance of postoperative bleeding, bile leak, or intraabdominal abscess.
In the LH group, the mean postoperative hospital stay was significantly shorter than in OH group, by an average of 4 days (p = 0.048). However, it must be emphasized that two patients in the LH group with postoperative ascitis prolonged the hospital stay to day 13 and 15, respectively, thus making the mean value for postoperative hospital stay significantly higher. For the patients without postoperative complications, the mean hospital stay was 7.4 ± 1.7 days (range, 5–11 days) for the LH group and 9.4 ± 2.8 days (range, 7–14 days) for the OH group (p = 0.043).
No patients were lost to follow-up evaluation. For the LH group, the mean follow-up period was 46.3 months. All the patients in both groups had at least a 24-month follow-up period.
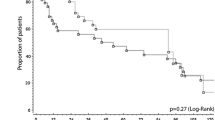
There were no differences in the middle-term outcome. In fact, the 2-year survival rate was similar in the two groups (Fig. 1). To date, no patient in either group has experienced recurrence at the site of resection, and no recurrence related to laparoscopy, such as peritoneal dissemination and port-site metastases, has been observed with LH.
Discussion
Hepatic resections performed for cirrhotic patients remains a surgical challenge for both the surgeons and the patients. The procedure has an acceptable mortality rate (5–10%) but a significant complication rate (10–40%) [2].
In the past decade, an increasing number of publications have reported on the laparoscopic treatment of benign liver tumors [7, 9, 14, 22, 23], but the number decreases if we consider HCC developed in a previous condition of liver cirrhosis, until recently considered a contraindication [7, 24]. Some recent reports [12, 25] have confirmed the technical feasibility and safety of the laparoscopic technique for cirrhotic patients with HCC, but an ideal prospective randomized study comparing open and laparoscopic resections has not been performed to date.
Our comparative study, even if retrospective, confirmed that laparoscopic hepatic resection for selected cirrhotic patients affected by small subcapsular HCC is an effective therapeutic option with a minor complication rate, a shorter hospital stay, and same oncologic results at 2 years compared with the traditional open approach. Although the small number of patients in each arm of the study and the retrospective nature of the control group makes the chance of a statistical error possible, we consider our conclusions reliable. The potential historical bias is reduced by the design of the study resulting in an OH group well matched with an LH group for age, gender, previous hepatitis infection, ASA class, tumor location and size, type of liver resection, and severity of liver disease.
The pre- and postoperative management of patients also was similar in the OH and LH groups. The selection criteria that we identified for a laparoscopic liver resection specified well-compensated chronic liver disease (Child-Pugh class A) without signs of severe portal hypertension (Oesophageal varices ≤ F2), exophytic or subcapsular tumors located in the left (II–III–IVb) or peripheral right (V–VI) segments, a maximum lesion size of 4 to 5 cm, and limited resection (<3 segments). Age, gender, and previous upper abdominal surgery cannot be considered risk factors, whereas complicated cirrhosis (Child-Pugh class B–C) and ASA class exceeding 2 must be considered effective contraindications. In fact, the only death in the entire study cohort occurred in the LH group (4.3%) and was caused by acute respiratory distress syndrome, which developed on postoperative day 3, probably due to a wrong preoperative evaluation of the respiratory function (ASA 3 instead of ASA 2).
Lesions sited to the left or peripheral right segments (II–VI, Couinaud) constitute a good indication allowing an excellent exposure of the whole operation field and a safe vascular control [26, 27]. Lesions of the posterior and superior liver segments (IVa, VII, VIII) are technically demanding, especially in terms of choosing the right resection plane and controlling bleeding [6, 8, 11, 12, 13, 16, 24]. In fact, in only one case (4.3%) were we compelled to convert to laparotomy just for a lesion located posteriorly in segment VII (but wrongly staged preoperatively in segment VI). A similar conversion rate is reported in other studies published recently [23, 24].
With regard to tumor size, we believe that lesions smaller than 5 cm could be treated laparoscopically via a limited resection (<3 segments). In our series, the mean tumor size was 3.1 cm (range, 1–3.9 cm) in the LH group and 3.2 cm (range, 1.6–4.2 cm) in the OH group. Larger lesions usually need a wider resection, but in our opinion, laparoscopic major hepatectomy, although possible, still is difficult and hazardous for cirrhotic patients, although a few cases have been reported recently in the literature [28, 29].
At the beginning of our experience, we selected only peripheral, subcapsular, or exophytic HCC that we still consider the best indication for a laporoscopic approach, especially in the early phase of the learning curve. Intraoperative ultrasound is mandatory and should always precede a planned operation aimed at hepatic resection in cirrhotic patients. The ultrasound allows correct staging of the tumor, precise evaluation of its extension including its relationships with major surrounding structures, and an oncologic free margin [30, 31]. As for open surgery, well-compensated cirrhosis (Child-Pugh class A) represents another essential factor in the performance of a laparoscopic liver resection.
All the patients in both groups had surgery by the same surgeon (G.B.), confirming the feasibility and safety of the laparoscopic approach when performed by a surgeon with extensive experience in hepatobiliary and advanced laparoscopic surgery. In fact, the mortality and the morbidity rates for the LH group were 4.3% and 13%, respectively.
The analysis of our results showed that the operative time was significantly longer for the laparoscopic procedure (148 vs 125 min; p = 0.01), but we must emphasize that in the last 10 patients, the operating time had been shortened to less than 2 h, confirming that laparoscopic surgery is a technique dependent on a learning curve [32].
The amount of bleeding in both groups was minimal. The blood loss was 260 ml in the laparoscopic group and 377 ml in the open group (nonsignificant difference). In the OH group, four patients (17.3 %) received blood transfusions, whereas no transfusion was needed in the LH group. Although there was no significant difference in blood loss between the two groups, more patients of the OH group received a transfusion because of an old anesthesiologic policy for perioperative management.
The difference in pedicle clamping between the two groups was statistically significant (p = 0.017), but this difference could be attributable to a different surgical policy in terms of vascular clamping applied during the last period in addition to the hemostatic effect of pneumoperitoneum. In any case, we think that this clamping, especially in laparoscopic resections for HCC on cirrhosis (mostly atypical resections), is not useful and could be not only tedious but dangerous to apply for large collateral veins and adhesions due to the severe portal hypertension associated with the underlying chronic liver disease.
Because the Pringle maneuver was not used for any of our patients in the LH group, the postoperative aspartate aminotransferase level did not rise above 100 U/l in 13 of our patients (56%), as noted by others [13, 16, 17]. Even the development of new surgical devices (e.g., Harmonic Scalpel, Ligasure Five) has greatly enhanced the safety of hepatectomy and improved control of bleeding during parenchymal transection performed either as an open procedure or laparoscopically [22, 33]. In fact, we never converted a procedure after uncontrolled hemorrhage.
The main clinical advantages of a minimally invasive technique used for liver resection in cirrhotic patients seem to be a significantly lower rate of postoperative complications (13% vs 47.8%; p = 0.010) and a consequently shorter postoperative hospital stay (8.2 vs 12.04 days; p = 0.048) both in complicated and noncomplicated patients. Postoperative ascitis is the most frequent complication of open hepatectomy among cirrothic patients, even for limited resections. In contrast, the laparoscopic approach, by avoiding a long abdominal incision and muscle division, reduces the risk of parietal hernias and, most important, preserves the wall portosystemic shunts, thus reducing the increase in portal hypertension and the consequent risk of postoperative bleeding and ascitis [5]. Further potential advantages include preservation of the round ligament, which may contain significant collateral veins, by less mobilization and manipulation of the liver and a decreased intraoperative fluid requirement [25]. In our LH series, ascitis developed in only three patients (grade 1, Dindo) compared with nine patients in the OH group (p = 0.043) (1 patient grade 3a).
Our results show that after laparoscopic resection, survival in the middle term was similar to that observed after open resection (LH 86.9% vs OH 82.6%). Clearance margin is the key to obtaining long-term survival after liver resection. As in the study of Gigot et al. [24], we observed no difference in the surgical margins between the two groups, although it was less than 1 cm for two patients in the LH group, confirming that clearance margin does not seem to affect oncologic results provided the resection is complete and the tumor is not exposed [13, 34]. In any case, we strongly recommend the use of intraoperative ultrasound not only to make a correct staging of the disease, but also to decrease the potential high risk of insufficient tumor clearance in laparoscopic procedures [31]. No local recurrences occurred in either group, confirming that the type of resection (anatomic vs nonanatomic) does not influence local postoperative recurrence rates [35]. Furthermore, in the LH group we had neither port-site metastases nor intraabdominal seeding nor other recurrences related to laparoscopy.
Finally, laparoscopic liver resection may be considered as a safe and effective bridge for patients with small HCC on a waiting list for orthotopic liver transplantation [36].
In conclusion the data of our series show the feasibility and safety of laparoscopic liver resection even for cirrhotic patients, and prove that laparoscopy offers a better postoperative course and a shorter hospital stay than the traditional open approach, with 2 good years of follow-up findings and better short-term results.
References
Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A (2001) Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg 234:71–78
Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J (2002) Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery 131:311–317
Gagner M, Rheault M, Dubuc J (1992) Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 6:97–98
Azagra JS, Goergen M, Gilbart E, Jacobs D (1996) Laparoscopic anatomical (hepatic) left lateral segmentectomy: technical aspects. Surg Endosc 10:758–761
Huscher CGS, Napolitano C, Chiodini S, Recher A, Buffa PF, Lirici MM (1997) Hepatic resections through the laparoscopic approach. Ann Ital Chir 6:791–797
Yamanaka N, Tanaka T, Tanaka W, Yamanaka J, Yasui C, Ando T, Takada M, Maeda S, Okamoto E (1998) Laparoscopic partial hepatectomy. Hepatogastroenterology 45:29–33
Marks J, Mouiel J, Katkhouda N, Gugenheim J, Fabiani P (1998) Laparoscopic liver surgery: a report on 28 patients. Surg Endosc 12:331–334
Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, Rotman N, Fagniez PL (2000) Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 232:753–762
Rau HG (1998) Laparoscopic liver resections compared with conventional partial hepatectomy: a prospective analysis. Hepatogastroenterology 45:2333–2338
Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL (2003) Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg 196:236–242
Morino M, Morra I, Rosso E, Miglietta C, Garrone C (2003) Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc 17:1914–1918
Belli G, Fantini C, D’Agostino A, Belli A, Russolillo N (2004) Laparoscopic liver resections for hepatocellular carcinoma (HCC) in cirrhotic patients. HPB 6:236–246
Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL (2003) Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 138:763–769
Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J (2002) Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepato-Biliary-Pancreatic Surg 9:242–248
Dulucq JL, Wintringer P, Stabilini C, Berticelli J, Mahajna A (2005) Laparoscopic liver resections: a single-center experience. Surg Endosc 19:886–891
Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T (2005) Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 189:190–194
Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K (2001) Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc 15:541–544
Belli G, Fantini C, D’Agostino A, Belli A, Langella S (2005) Laparoscopic hepatic resection for completely exophytic hepatocellular carcinoma on cirrhosis. J Hepatobiliary Pancreat Surg 12:488–493
Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW (2000) The Brisbane 2000 terminology of liver anatomy and resections. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB 2:333–339
Belli G, Fantini C, D’Agostino A, Belli A, Cioffi L, Russolillo N (2006) Laparoscopic left lateral hepatic lobectomy: a safer and faster technique. J Hepatobiliary Pancreat Surg 13:149–154
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and result of a survey. Ann Surg 240:205–213
Belli G, Fantini C, D’Agostino A, Belli A, Russolillo N, Cioffi L (2005) Laparoscopic liver resection without Pringle Maneuver for HCC in cirrhotic patients. Chir Ital 57:15–25
Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, Morino M, Bismuth H, Castaing D, Savier E, Honore P, Detry O, Legrand M, Azagra JS, Goergen M, Ceuterick M, Marescaux J, Mutter D, de Hemptinne B, Troisi R, Weerts J, Dallemagne B, Jehaes C, Gelin M, Donckier V, Aerts R, Topal B, Bertrand C, Mansvelt B, van Krunckelsven L, Herman D, Kint M, Totte E, Schockmel R, Gigot JF (2003) Laparoscopic liver resection of benign liver tumors: results of a multicenter European experience. Surg Endosc 17:23–30
Gigot JF, Glineur D, Azagra JS, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, Descottes B, Valleix D, Lachachi F, Bertrand C, Mansvelt B, Hubens G, Saey JP, Schockmel R (2002) Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 236:90–97
Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL (2006) Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg 243:499–506
Belli G (2004) Symposium on laparoscopic liver surgery: editorial. HPB 6:195–196
Chang S, Laurent A, Tayar C, Karoui M, Cherqui D (2007) Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 94:58–63
Vibert E, Perniceni T, Levard H, Denet C, Shari NK, Gayet B (2006) Laparoscopic liver resection. Br J Surg 93:67–72
O’Rourke N, Shaw I, Nathanson L, Martin I, Fielding G (2004) Laparoscopic resection of hepatic colorectal metastases. HPB 6:230–235
Lo CM, Lai EC, Liu CL, Fan ST, Wong G (1998) Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg 227:527–532
Santambrogio R, Opocher E, Ceretti AP, Barabino M, Costa M, Leone S, Montorsi M (2007) Impact of intraoperative ultrasonography in laparoscopic liver surgery. Surg Endosc 21:181–188
Kaneko H (2005) Laparoscopic hepatectomy: indications and outcomes. J Hepatobiliary Pancreat Surg 12:438–443
Schmidbauer S, Hallfeldt K, Sitzmann G, Kantelhardt T, Trupka A (2002) Experience with ultrasound scissors and blades (Ultracision) in open and laparoscopic liver resection. Ann Surg 235:27–30
Poon RT, Fan ST, Ng IO, Wong J (2000) Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg 231:544–551
Kyung–Suk Suh (2005) Systematic hepatectomy for small hepatocellular carcinoma in Korea. J Hepatobiliary Pancreat Surg 12:365–370
Poon RTP, Fan ST, Lo CM, Liu CL, Wong J (2002) Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function. Ann Surg 235:373–382
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belli, G., Fantini, C., D’Agostino, A. et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc 21, 2004–2011 (2007). https://doi.org/10.1007/s00464-007-9503-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9503-6