Abstract
The efficacy of various lithium chloride (LiCl) applications in eradicating the parasitic mite Varroa destructor in honey bee colonies was investigated, with a specific focus on its impact on brood development. In broodless colonies (3 weeks post queen caging), the highest efficacy of 98% was achieved with a 9-day treatment of 2.5 kg of candy spiked with 50 mM LiCl. A shorter 5-day treatment with 2 kg of 50 mM LiCl candy resulted in an efficacy of 78%. In colonies with brood, a repeated short-term application of 4 × 0.5 kg 50 mM LiCl candy yielded an efficacy of 88%. LiCl treatment led to a removal of the first batch of brood reared after release of the queen. However, no long-term effects on colony growth were observed, and the colonies successfully overwintered. Additionally, the study demonstrated that lithium is rapidly distributed among the bees of a colony within 2 days, yet only low concentrations were detected in stored food samples. This suggests that the bees efficiently absorb and distribute lithium within the colony. The harvested honey in the following spring revealed a lithium concentration of 0.1–0.2 mg/kg, which is below naturally occurring lithium levels in honey. Based on these findings, LiCl can be considered an effective and easy-to-apply acaricide in broodless colonies, and even in colonies with brood, it had good efficacy and no long-term effects on colony survival. Further research may be necessary to determine the optimal treatment period for achieving an efficacy over 95%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The negative impacts from globalization are exemplified by the worldwide spread of the honey bee mite Varroa destructor (Anderson and Trueman 2000), a striking example of the redistribution of diseases and parasites in wildlife and livestock (Travis et al. 2011). V. destructor is originally a parasite of the Eastern honey bee Apis cerana (Fabricius 1793) in Asia. Intensive trade of managed Apis mellifera (Linnaeus 1758) colonies during the last century enabled the mite’s shift to this new host and promoted worldwide distribution (Chantawannakul et al. 2016; Chapman et al. 2023).
V. destructor causes severe morbidity and mortality of entire A. mellifera colonies by feeding on host tissues and transmitting viruses (Rosenkranz et al. 2010; Traynor et al. 2020). Regular Varroa treatments are indispensable because without beekeeper intervention, colonies dwindle (Genersch et al. 2010; Gray et al. 2019; Hernandez et al. 2022; Seitz et al. 2015; Stahlmann-Brown et al. 2022). Colony losses due to Varroa infestation continue to be high for two main reasons: firstly, there has been limited success in breeding viable Varroa-resistant honey bees (Büchler et al. 2010; Mondet et al. 2020a, b) and secondly, the biology of V. destructor with the reproductive phase inside the protected brood cell hinders the effectiveness of treatments (Traynor et al. 2020). When brood is present in the colonies, reproductive mites are located within the capped brood cells (Fuchs 1985, 1992; Ifantids 1988) and thus protected against nearly all common acaricides, except formic acid (Rosenkranz et al. 2010). A further challenge to controlling V. destructor under temperate climate conditions is that (i) chemical treatments should be avoided during nectar flows when bees collect nectar to make honey yet (ii) a treatment must be performed in late summer before the bees rear their long-lived winter bees, so that their longevity is not compromised through high levels of parasitization. High mite infestation levels in late summer and the subsequent damage to winter bees, as mite levels continue to be high in fall, are the main drivers of winter colony losses (Genersch et al. 2010; Gray et al. 2019; Seitz et al. 2015). The relatively small window of time for late summer treatment increases the need for highly effective varroacides in mite management strategies. Several varroacides are registered; however, the majority are based on only a few active compounds such as formic and oxalic acid, thymol and a few synthetic substances from the group of pyrethroids, formamidine, and organophosphate (Mutinelli 2016; Qadir et al. 2021; Vilarem et al. 2021). None of these registered products fulfill all desired characteristics of an “ideal” veterinary drug: (1) high and reliable efficacy, (2) limited side effects, (3) no residues in bee products above critical thresholds, (4) low risk of mite resistance, and (5) easy-to-apply. The widely used organic acids are poorly tolerated by bees or brood with significant impacts on colony size, especially in warm climates (Bubnič et al. 2021; Elzen et al. 2004; Rademacher et al. 2017; Satta et al. 2005). In particular, formic acid, a common agent used in late summer as it penetrates into the brood nest, requires specific environmental conditions and its efficacy is strongly influenced by colony factors, such as colony size and amount of colony thermoregulation (Pietropaoli and Formato 2019; Steube et al. 2021; Underwood and Currie 2003). Repeated applications are necessary to achieve sufficient efficacy, which makes the use of organic acids time-consuming and thus costly, especially in large beekeeping operations (Berry et al. 2022). Synthetic varroacides often result in accumulating levels of residues in beeswax, with potential impacts on later larval survival and queen quality (Albero et al. 2023; Haarmann et al. 2002; Kast et al. 2021), and frequent reuse leads to the development of mite resistance (Higes et al. 2020; Mitton et al. 2022). Due to these shortcomings of the current options for chemical control of Varroa, there is still urgent demand for further varroacidal compounds.
The recently discovered lithium chloride (LiCl) meets many of the above mentioned requirements of a varroacide (Ziegelmann et al. 2018), and so we wanted to examine its efficacy and distribution in free flying colonies. LiCl is a widely distributed salt (Szklarska and Rzymski 2019), a natural component of honey (Abdulkhaliq and Swaileh 2017; Bogdanov et al. 2008; Conti et al. 2018; Karabagias et al. 2017; Tariba Lovaković et al. 2018), and is used in therapeutic treatments of bipolar disorders and depression (Ferensztajn-Rochowiak et al. 2021; Gomes-da-Costa et al. 2022). LiCl is very effective at killing mites via contact and displays a systemic mode of action, while being well-tolerated by adult honey bees (Kolics et al. 2020, 2021b; Ziegelmann et al. 2018). A limitation for a broad applicability of LiCl is currently the low tolerability by honey bee brood (Rein et al. 2022).
Climate change alters the way beekeepers manage colonies, as often colonies no longer have a natural brood break which enables an effective winter treatment. In southern Europe, it has therefore become common to cage queens after the summer honey harvest and then treat the mites in the dispersal phase on adult bees when colonies are broodless (Büchler et al. 2020; Lodesani et al. 2014). LiCl is an ideal compound under these circumstances, due to its high efficacy and good tolerability by adult bees (Stanimirovic et al. 2022; Ziegelmann et al. 2018). Some field tests with lithium salts have already been performed; however, most of them used a repeated trickling method similar to the application of oxalic acid (Jovanovic et al. 2022; Kolics et al. 2021b, 2022).
In our approach, we wanted to capitalize on the systemic mode of action of LiCl, i.e. the administration of the active substance via food to the colonies. This new application method would enable a quick and “easy-to-apply” treatment. We present the efficacy and distribution of LiCl using different food applications in broodless colonies under realistic field conditions with a special focus on the subsequent development of the brood. In the trial of 2022, we compared the broodless application with repeated short-term treatments in brood rearing colonies. We hypothesized that such short-term treatments would reduce the exposure time and risk of honey bee larvae to LiCl and therefore potentially reduce the loss of brood as described in Rein et al. (2022).
Materials and methods
Experimental setup
Field trials were performed in two apiaries. The preliminary experiment in 2018 took place at the field station Heidfeldhof of the University of Hohenheim (48°42′55.3″N 9°10′49.2″E), whereas the main experiments in 2021 and 2022 were performed in the local apiary of the State Institute of Bee Research at the University of Hohenheim (48°42′32.7″N 9°12′38.9″E). Each colony consisted of a total of 20 frames in two Zander size brood boxes with similar colony strength—estimated by the number of combs covered with bees and the number of brood combs—and headed by healthy sister queens. The queens originated from the local “wildtype” stock managed by the State Institute of Bee Research, University of Hohenheim, Stuttgart, Germany.
The experimental setups of the different trials are shown in Table 1. Our main objective was to investigate different applications of LiCl in broodless colonies during summer, so to achieve broodless colonies we first had to cage the queen. After the last honey harvest in summer, the queen of the respective colony was placed in a small plastic cage (Varroa control box, Rubee®) on a frame in the middle of the brood nest in the bottom hive body at the start of each trial (Fig. 1). The cages are designed with queen-excluder sized material, allowing worker bees to pass through while confining the queen. After three weeks, when the last of the brood emerged, the queen of the respective colony was released and the treatment with LiCl started on the same day.
In the preliminary experiment, we tested two different LiCl applications with 25 mM LiCl syrup and 50 mM LiCl candy and compared the efficacy to positive control colonies treated with 250 ml formic acid (60%, Nassenheider® evaporator) (Table 1). The concentrations were chosen based on previous experiments by Ziegelmann et al. (2018) with artificial swarms.
In the main experiments, we used 50 mM LiCl candy, as it was the more promising approach due to ease of application combined with a slower, more consistent consumption of the LiCl food. In 2021, we tested a single application (A1) of 2.5 kg 50 mM LiCl candy (fed over a period of 9 days via a plastic bowl protected by an empty honey chamber) and compared it with the positive control group, treated with 250 ml formic acid (60%, Nassenheider® evaporator) (Table 1). In 2022, we modified the LiCl application and fed 2 kg 50 mM LiCl candy via small Ziplock plastic bags (1 l SafeLoc®, Toppits®) put directly on top of the frames (A2) (Fig. 2), due to ease of application and to provide ready access for the bees. We also shortened the treatment period from 9 to 5 days to eliminate exposure of the newly developed larvae with LiCl. A flipped top feeder (Nicot®) provided enough space for the bees to access the treatment food.
To test whether a repeated short-term application (A3) can prevent brood removal with the same Varroa control efficacy, we applied 0.5 kg 50 mM LiCl candy four times in 7-day intervals (in total 2 kg candy) without caging the queen, meaning that all brood stages were present during the treatment. The plastic bags containing 0.5 kg candy were emptied within 2 days of application, therefore the treatment period was defined as 4 × 2 days. Untreated colonies were not used as a negative control to avoid mite reinfestation by highly infested nearby colonies (Frey and Rosenkranz 2014), which would result in inaccurate calculations of efficacy.
Data were collected on Varroa mite mortality, brood development, and distribution of lithium in sampled bees and stored food as described below.
LiCl food production
For the syrup, we used Apiinvert® (saccharose, fructose and glucose, Suedzucker Group©) and mixed it with the respective amount of lithium chloride (> 99.9%, p.a., ultra-quality, Roth®) to reach a 25 mM concentration (1.06 g LiCl-salt per liter). We added food coloring (“Tannengrün”, Städter®) to the syrup, which dyed it green, so we could identify where the syrup was stored in the combs. Such food coloring has no negative impact on honey bees or honey bee brood (Ehrenberg et al. 2019).
The candy was made from powdered sugar (Suedzucker Group©) and honey harvested during summer nectar flows, mixed in the ratio of 2:1. Additionally, a concentration of 50 mM LiCl and red food coloring (Allura Red AC, Sigma-Aldrich®) was added into the mixture. To reach this concentration, we used 1.57 g LiCl-salt dissolved in water for 1 kg of candy. This was mixed until the food coloring was evenly distributed using a commercial dough mixer (ITR50 2 V “Evo”, Prismafood DE). To determine the exact amount of LiCl used per colony, food consumption was measured by weighing the remains of the applied food upon removal.
Varroa mite mortality and calculation of efficacy
The mite mortality was recorded by counting the mites every two days on the sticky board inserted under the screened bottom board of the hive, which was not accessible to bees. The sticky board is a tray prepared with a single layer of kitchen paper towel, moistened with oil, which prevents other insects from removing the dead mites and which was renewed after each mite mortality count. The natural mite drop was recorded two weeks prior to the treatments. Dead mites found on the sticky board during LiCl food application and the consecutive 7 days were considered killed by LiCl. For the positive control colonies treated with formic acid, we considered dead mites on the sticky board from 1st to 20th day after treatment administration. Formic acid penetrates the sealed brood cells to kill mites inside capped cells, which is why we extended the monitoring period to cover an entire brood cycle.
Each colony received a follow-up treatment for four to six weeks with either Bayvarol® (2018 and 2022) or Apivar® (2021). We alternated the compound for the follow-up treatments every year to avoid development of mite resistance. In addition, the good efficacy of both, Bayvarol® and Apivar® has been confirmed by field trials conducted on the Hohenheim campus in the previous years.
The efficacies of the treatments were calculated on the basis of mite mortality in the test colonies. The following formula was used according to standard guidelines for control of V. destructor (Dietemann et al. 2013; EMA 2021; Pietropaoli and Formato 2019; Semkiw et al. 2013), where the number of mites killed by the treatment is divided by the total number of mites that fell including those killed by the follow-up treatment:
Honey bee brood survival
To evaluate the effects of the different LiCl treatments on brood, we conducted a brood assessment of newly laid eggs in the main experiment. We marked the position of eggs on a transparent acetate sheet (= brood area fixing day (BFD)), according to the method described by Schur et al (2003). We inspected the viability of the brood every four days and marked cells with viable larvae as well as cleared out cells on a new transparent sheet. As eggs do not hatch for three days, the exact age of the larvae varied between 1 and 72 h. To keep the error rate low and to guarantee that empty cells were cleared out and the bees had not emerged, we terminated the assessment on day 16 (BFD + 16). From these data, we calculated the brood survival rate for each assessment.
After being caged for three weeks, the queen typically requires a few days to start laying eggs again, therefore we started the brood assessment in treatment group A1 five days after the release of the queen and the start of the treatment application. In treatment group A2, we were already able to find enough eggs three days after queen release. In 2022, we not only examined the development of the first brood cycle, but also a second brood cycle 16 days later to evaluate possible long-term effects (Table 1) for both the A2 and A3 treatment groups.
Distribution of lithium among worker bees, stored food, and residue in honey
An even distribution of the active component lithium among the worker bees is important for a sufficient treatment efficacy. In contrast, the chloride anion is not significant (Ziegelmann et al. 2018). Thus, we utilized ICP-MS analysis to determine the concentration of lithium in the samples. In 2021, we took bee samples (approx. 30 bees) from each treated colony at regular intervals during and post treatment (as shown in Online Resource 1). In 2022, we took bee samples from the central frames of the hives every other day for three weeks in trial A2 and for four weeks in trial A3, as well as collecting additional bees 39 days post-treatment from both groups. Stored food samples were taken once a week until day 39 post-treatment from four combs containing food stores in the top hive body.
To investigate whether the LiCl treatments had an effect on the lithium levels in food and honey of the following year, we took a sample from the brood chamber of each colony before installing the honey chamber on 19th of April 2023. Three weeks later, we took samples from the honey chamber of each colony that already collected nectar. On the 9th of June, the honey was harvested for each treatment group individually (A2 and A3) and samples were analyzed.
After sampling, the bees were frozen (− 20 °C) until dissection of the honey crop. Bees were thawed, and we gently pulled on the abdomen with forceps, which exposes the crop. The crops of 20 bees per sample were pooled, homogenized, and analyzed with ICP-MS (Inductively Coupled Mass Spectrometry) for the concentration of lithium. The samples of stored food from the colony were also frozen (− 20 °C) until analysis.
For ICP-MS analysis, about 500 mg of honey or crop samples, respectively, was weighed into glass tubes. 2 ml of HNO3 was added, and tubes were filled up with double distilled water to a final volume of 10 ml and vortexed to ensure sample homogeneity. Following this, microwave digestion was done with an Ultra Clave III (MLS Mikrowellen-Labor-Systeme GmbH, Leutkirch, Germany) where temperature was gradually increased from 80 to 200 °C at 900 W and 100 bar. Samples were cooled down and diluted adequately for ICP-MS analysis (NexION 300 X, PerkinElmer, Waltham, MA, USA). A ICP multi-element standard solution (Merck KGaA, Darmstadt, Germany) was used for calibration at concentrations of 0.1 µg/l, 0.2 µg/l, 1 µg/l, 10 µg/l, and 20 µg/l prepared with double distilled water and HNO3. A CertiPUR Rhodium ICP-standard solution (c = 1000 mg/l Rh, Merck KGaA, Darmstadt, Germany) served as internal standard (LOQ < 0.025 mg/kg).
Statistics
The data were statistically analyzed using JMP Pro 16. Data on efficacy were checked with a Shapiro–Wilk test for normal distribution. Because of the non-parametric characteristics of the data, we used the Kruskal–Wallis test and a post hoc Dunn’s test including Bonferroni correction for multiple comparisons. For comparison of the 1st and 2nd brood assessments of each treatment we used the Wilcoxon Rank Sum test (Mann–Whitney test).
Results
Efficacy of different treatments
In the preliminary experiment in 2018, the broodless colonies treated with LiCl demonstrated an efficacy of 92.0 ± 4.5% for the syrup application (P1) where each colony received 7 l of 25 mM LiCl spiked Apiinvert®. For the candy application (P2), we applied 4.5 kg 50 mM LiCl which resulted in a mean food uptake of 2040 ± 786 g of candy per colony in 8 days (additional data are given in Online Resource 2) and an efficacy of 89.8 ± 5.6%, with no significant difference between the treatments (Fig. 3, p = 1, Dunn’s test, Online Resource 3). Treatment with LiCl outperformed the positive control treatment with formic acid, where only 59.5 ± 8.5% of the mites were killed. The formic acid treated colonies did not have caged queens, as the treatment penetrates into the brood nest, and we wanted to compare LiCl results with the most commonly used method of Varroa control in Germany.
Efficacy of different treatments in the preliminary trials 2018 shown as box plots and mean indicated by the x. Positive control colonies were treated with 250 ml 60% formic acid (n = 4), P1 colonies were treated with 7 l of 25 mM LiCl syrup (n = 4), P2 with 4.5 kg 50 mM LiCl candy (n = 5). Significant differences are indicated by different letters (p < 0.05, Dunn’s test; additional data are given in Online Resource 3)
In the years 2021 and 2022, LiCl was exclusively applied via candy (Fig. 4). For treatment A1 each colony received 2.5 kg 50 mM LiCl candy, which resulted in a mean food uptake of 1,739 ± 167 g in 9 days and the highest efficacy with 98.1 ± 0.7%, followed by A3 with 87.9 ± 10.5% and A2 with 77.5 ± 12.8%. For treatment A2 and A3 each colony consumed a total of 2 kg of 50 mM LiCl candy, which incorporates 3.1 g LiCl-salt (additional data are given in Online Resource 2). Positive control with formic acid was the least effective (69.8 ± 25.2%) and showed the highest variation between colonies, ranging in efficacy from 22.4% to 96.9%. Due to higher variances in A2 and A3, they did not differ significantly from the positive control (p = 1 and p = 0.6, respectively, Dunn’s test, Online Resource 4) (Fig. 4).
Efficacy of different LiCl administrations and positive control shown as box plots and mean by x. Positive control colonies (n = 9) were treated with 250 ml 60% formic acid. For A1 (n = 6) we applied 2.5 kg 50 mM LiCl candy over a period of 9 days; A2 colonies (n = 10) received 2 kg 50 mM LiCl candy in 5 days; for A3 (n = 10) we applied 0.5 kg of 50 mM LiCl candy four times in seven-day intervals (total of 2 kg candy) without caging the queen. Box plots with different letters are statistically different (Dunn’s test, p < 0.05, additional data are given in Online Resource 4)
The total number of counted mites in 2021 showed a mean mite load per colony of 3,087 ± 915 for positive control colonies and 2,129 ± 967 for A1 colonies. Each colony had more than 1000 mites (Table 2). In 2022, the mean mite loads were on average 1429 ± 1155 mites for the A3 colonies and substantially lower in the A2 colonies with on average 456 ± 296 mites. Here, the lowest number of total counted mites per colony was 153 and 168, respectively.
The mean number of mites killed per colony by the follow-up treatment was 933 in positive control colonies, 36.3 in colonies treated with A1, 83.1 in those treated with A2, and 95.9 for those treated with A3 (Table 2). This information can be used for assessing whether the LiCl treatments alone have reduced the mite population below the economic threshold.
Honey bee brood survival
In total, we assessed 9463 brood cells from the freshly laid egg stage in 6 colonies of A1, 8 colonies of A2 and 10 colonies for both brood assessments of A3. Due to absence of eggs in two colonies on the assessment day, we could only examine the brood development for 8 of 10 colonies in A2.
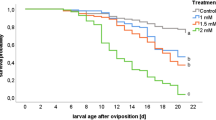
Treatment A1 with 9 days of LiCl treatment led to the highest brood removal rates and only 4.7% of the brood survived until BFD + 16 (Fig. 5). The first assessment of A2 (A2-1) showed a high variability in the survival probability of brood from the 8 treated colonies with a mean brood survival rate of 45%. The 2nd assessment (A2-2), which started 19 days after the LiCl application, had the highest brood survival rate with 82%, which was statistically different to the first assessment (p = 0.02, Wilcoxon Rank Sum test). The mean brood survival rate of the first assessment in A3 was 52% and thus similar to the first assessment of A2. However, in the 2nd brood assessment (A3-2), which started 2 days after the 3rd LiCl application, the brood survival rate decreased to only 8%, which was significantly lower than A3-1 (p = 0.004, Wilcoxon Rank Sum test).
Brood survival rates in free flying colonies during different applications of 50 mM LiCl candy. A1: 2.5 kg of 50 mM LiCl candy over 9 days (n = 6); A2: 2 kg of 50 mM LiCl candy over 5 days (n = 8); A3: 4 x 0.5 kg 50 mM LiCl candy in seven-day intervals in colonies without queen caging (n = 10). For treatment A2 and A3, a subsequent brood assessment was carried out after completing the first one. Data given as boxplots for survival rate on day 16 post the brood fixing day (BFD), mean is given as an x. Total number of selected eggs on BFD were 982 (A1), 2574 (A2-1), 1600 (A2-2), 2049 (A3-1) and 2258 (A3-2). Significant differences between 1st and 2nd assessments are indicated with an asterisk (Wilcoxon Rank Sum test)
Colony winter survival
In winter 2021, we lost one colony from the positive control group due to Nosema disease. The diagnosis was made through microscopic examination of sampled bees. In the 2022 experiments, we found one colony dead in the following spring probably due to queen loss in fall. In this colony, the queen was still present during the first brood assessment (A2-1); however, during the second assessment (A2-2), we were not able to find eggs. All the other colonies overwintered well.
Distribution and detection of lithium
In colonies treated over a period of nine days (A1), we took 66 stored food and 61 adult bee samples over a period of 21 days. The 1st sampling was conducted before LiCl application, showing no lithium contamination in bees nor in food (Online Resource 5). Within five days of commencing the treatment, the concentration of lithium in the honey crop of bees sampled from the central area of the lower hive body rose to 69 mg/kg lithium and doubled four days later to reach the maximum of 131 mg/kg. After the remaining LiCl food was removed on day 9, the lithium concentration quickly dropped through day 17; thereafter it increased slightly during the last sampling 21 days post treatment. On no sampling day was there a significant difference between the lithium concentration from bees sampled from top or bottom hive body in our two brood chamber set-up. The increase in the lithium concentration in the honey crop was mirrored by the number of fallen mites. The mite drop reached a maximum on the fifth day, when a mean of 983 mites dropped, decreasing thereafter as shown in the figure (Online Resource 5).
In the stored food samples, we first detected lithium in uncapped cells on day 9 after commencing the LiCl treatment (25 mg/kg), whereas the first detection in capped cells was on day 13 (4.4 mg/kg). The lithium concentration was always lower in samples taken from capped cells and was significantly lower on day 17 with 5.2 mg/kg lithium compared to 36 mg/kg in open cells (p = 0.013, Tukey–Kramer HSD) (Online Resource 5).
In 2022, when colonies were treated for five days (A2), 130 bee samples were taken from the treated colonies. From the colonies repeatedly treated for 4 × 2 days (A3) 170 bee samples were taken. Lithium concentration in the honey crop of bees from the A2 treatment displayed a similar distribution as in A1 group with a peak of 104 mg/kg lithium on day 3, decreasing after termination of treatment (Fig. 6). Maximum lithium concentration in stored food occurred 13 days after the start of the treatment (18 mg/kg).
Distribution of lithium in the honey crop of sampled bees and in stored food from colonies (n = 10) during the five-day treatment (A2), each value given as the mean ± SE in the error bars. Administration of 2 kg of 50 mM LiCl candy started 1 day after the first sampling and was terminated five days later. The mean number of fallen mites for every two days is shown by the orange line. Follow-up treatment with Bayvarol® started on day 11
For the repeated short-term treatments used in A3, we detected several short peaks after each application of the 0.5 kg LiCl candy (Fig. 7). The highest concentration in the honey crop was found on day 16 after the 3rd application with 98 mg/kg lithium. The concentration of lithium in the sampled food reached a maximum on day 28 with 20 mg/kg. For both treatments, A2 and A3, we took the last samples 39 days after the last application of LiCl and measured 15.4 mg/kg lithium in the A2-treated colonies and 13.2 mg/kg in the A3-treated colonies.
Distribution of lithium in the honey crop of sampled bees and stored food from colonies (n = 10) during the repeated short-term treatment (A3), each value given as the mean ± SE in the error bars. Administration of each 0.5 kg 50 mM LiCl candy took place on days 0, 7, 14, and 21. The mean number of fallen mites for every 2 days is shown as the orange line. Follow-up treatment with Bayvarol® started on day 30
The stored food sampled from brood chambers in Spring 2023 revealed low concentrations of 5.4 ± 2.4 mg/kg lithium in A2-treated colonies and an even lower concentration of 3.1 ± 2.4 mg/kg in the A3 treatment group (Table 3). In the freshly collected nectar from honey chambers, we measured 0.8 ± 0.7 mg/kg lithium from A2 colonies, which was further reduced to 0.1 mg/kg in the harvested honey. For A3, we measured 0.2 ± 0.2 mg/kg and 0.2 ± 0.2 mg/kg, respectively (Table 3).
Discussion
Our field experiment demonstrated high efficacy of LiCl in colonies with and without brood when applied by feeding in syrup or candy. In the preliminary experiments in the year 2018 with broodless colonies, we already reached a mean efficacy of more than 90% with both the syrup and the candy application. We then decided to continue with the candy treatment, because of the slower consumption by the bees which enables a continuous flow of LiCl into the colony for more than one week through a single feeding. The field test with broodless colonies in the year 2021 revealed an even higher efficacy of 98%, which was significantly different from colonies treated with the standard formic acid Varroa control in the same apiary. This confirmed that the high efficacy of LiCl, which had previously been demonstrated by Kolics et al. (2021b) with the trickling method, can also be achieved using a single application of 2.5 kg candy spiked with 50 mM LiCl. The next field test with broodless colonies in the year 2022 had only a 78% efficacy. Two reasons could potentially have caused this significantly lower efficacy compared to the 2021 study. First, we reduced the duration of the treatment from 9 to 5 days to reduce the impact on brood loss in the first subsequent brood cycle seen in the 2021 experiment (see below). But this shortened period was obviously not sufficient to achieve a high efficacy. Second, the mean mite load per colony was 456 mites compared to 2129 in the year before and some colonies had less than 200 mites. In colonies with relatively low mite numbers, the reinvasion of mites in late summer and early fall (Frey and Rosenkranz 2014), a common occurrence in apiaries in this part of Germany, can have a greater impact on the calculation of the efficacy. Our experimental apiaries were not in the direct vicinity of colonies managed by other beekeepers, however even over a distance of 1.5 km an invasion of more than 100 mites per colony is possible (Frey et al. 2011).
Our repeated short-term LiCl treatments of colonies with brood revealed an efficacy of 88% and confirmed the results of former field tests with lithium salts in brood rearing colonies (Stanimirovic et al. 2022), however at the cost of high brood removal rates (see below). The efficacy of a treatment is not the only important value, rather it is crucial whether the LiCl treatments reduce the absolute number of mites in all colonies below the economic threshold. This economic threshold can be estimated in various ways such as the natural daily mite drop or the infestation in sampled adult bees. Jack and Ellis (2021) defined an infestation rate of the adult bees of 2–3% as a general accepted economic threshold. In our follow-up treatments directly after the LiCl applications we killed an average of only 36–96 mites per colony. With an average number of about 15,000–20,000 bees in our colonies at the end of September, this number of mites represents an infestation of considerably less than 1%. Therefore, we consider LiCl applications in late summer as sufficiently effective to reduce the Varroa infestation below the economic threshold, providing beekeepers with a highly effective alternative treatment option once it is approved for use.
Our analysis of honey bee crops and stored food for traces of lithium demonstrated an even distribution of the active ingredient lithium within the colony, despite food uptake differing somewhat between colonies. This low variance between colonies in the distribution of the active ingredient lithium and the efficacy of treatment results in high levels of treatment success throughout an apiary, which cuts down on varroa reinvasion and potential transfer of mites between strong and weak colonies (Giacobino et al. 2023).
In contrast to currently available varroacides, our application method of LiCl takes advantage of the social behavior of food sharing in bees, called trophallaxis (LeBoeuf 2017). This leads to a rapid distribution of the treatment food throughout the whole colony (Crailsheim 1998; Nixon and Ribbands 1952) with sufficient amounts of lithium to kill Varroa mites in sampled bees within 48 h of application, as demonstrated by the spike in the mite drop within two days of LiCl administration (see Fig. 6). We always detected substantially lower levels of lithium in the food samples than in the crop of the sampled bees. In 2021, when about 1.7 kg of a 50 mM LiCl candy was applied per colony, the maximum lithium content found in open food cells was 36 mg/kg, whereas in the crop it rose to 131 mg/kg. In 2022, when the feeding period was reduced from 9 to 5 days, the maximum lithium levels were somewhat lower, with similarly low levels in food compared to bee crops as seen in 2021. To produce honey from nectar or from a beekeeper applied food source like syrup or candy, honey bees rework the food source multiple times, before permanently storing it in a cell (Park 1925). During this food processing, the bee’s ventriculus removes contaminations and excess water. Honey bees also have the capacity to remove heavy metals from collected nectar in the process of producing honey (Borsuk et al. 2021), which could help explain why we always detected substantially lower lithium concentrations in stored food compared to crop samples.
As shown in previous studies, exposure to LiCl during larval development can result in high brood mortality (Rein et al. 2022). We thus paid particular attention to the survival of the first batch of brood reared after release of the queen. In 2021, the long treatment period of 9 days resulted in low brood survival, most likely due to an overlap of applied LiCl food and the presence of the first larvae. To avoid this loss of brood, we shortened the treatment period to 5 days in 2022, reducing the exposure of the sensitive early larval stages to LiCl. Brood survival increased, but with high variance in survival rates among the colonies. We assessed a second brood cycle, which showed that there are no long-term effects to brood survival from LiCl as was also observed in 2021. This is also supported by the fact that 34 colonies treated with LiCl (from all experiments) overwintered well and only one colony died due to queen loss in fall 2022. Thus, although we see a short time frame of brood loss immediately after treatment, the colony then successfully rears brood and does not suffer any population loss from this brief interruption.
Other studies tested different LiCl applications in broodless colonies with even higher concentrations, but failed to evaluate the aftereffects on brood reared post treatment (Kolics et al. 2022). Stanimirovic et al. (2022) carried out lithium citrate treatments in colonies with brood and stated that there were no side effects observed during the year, however the authors did not analyze potential carryover effects of lithium citrate on brood directly after the treatment applications. Our experiments with repeated short-term treatments in colonies with a free-roaming queen and brood (A3) clearly confirm the low tolerability of honey bee larvae for LiCl. The goal with this new application method was to limit the contact of larvae to LiCl for a maximum of 1–2 days by feeding the treatment repeatedly in small amounts. However, this did not provide the desired reduction in brood loss. Both the first and second brood assessment during application resulted in low survival rates and so clearly a treatment with LiCl in colonies with brood is only possible at the cost of lost brood during the application period. As seen in our studies, the side effects on brood only occur during and shortly after the period of the treatment when lithium contaminated food is still circulating among the nurse bees.
From our comprehensive studies on LiCl, we can conclude the following regarding efficacy, distribution of the compound within the colony and side effects on brood for the treatment of broodless colonies:
-
1.
A single feeding of 2 kg of 50 mM LiCl candy kills more than 95% of Varroa mites under field conditions when exposure lasts 9 days. It could potentially be shortened, but 5 days was too short to achieve the required 90% efficacy for Varroa mite control.
-
2.
Side effects on the honey bee brood occurred only in the first brood cycle laid directly after the end of the application. If queens were released one week after treatment cessation, these side effects could largely be prevented.
-
3.
Our data on the concentration and distribution of lithium within the worker bees of a treated colony indicate that within 48 h lithium levels reach their maximum level within the bee’s crop and remain elevated for the duration of the treatment. Even some days after the end of the application the lithium concentrations within the crops of the bees should still be high enough to kill the parasitizing mites.
These results suggest that a promising strategy for future applications would be to start the treatment via candy one week before the release of the queen, when the colony only contains capped brood. The lithium concentration in the bees at the time of queen release should be sufficient to kill the few mites emerging with the last brood cells, yet subside to a harmless level by the time the first larvae hatch from queen laid eggs.
The long-term circulation of lithium in colonies post treatment and thus the potential risk of residue accumulation in the honey must be investigated before lithium-salts can be authorized as a varroacide. Lithium occurs naturally in some honeys (up to 15.6 mg/kg) (Abdulkhaliq and Swaileh 2017; Bogdanov et al. 2008; Conti et al. 2018; Tariba Lovaković et al. 2018), mineral water (1.7–1725 µg/l) (Seidel et al. 2019), and even other beverages like wine and soft drinks (Seidel et al. 2020). We even found lithium at a concentration of 0.27 mg/kg in the Apiinvert® syrup sold as bee feed on the German market. It is thus hard to define an acceptable residue level, though the naturally occurring range in honey suggests a higher limit than with other varroacide residues is warranted. What is considered acceptable is currently debated; Kolics et al. (2021a) found an increase in lithium in uncapped honey directly after application but claimed a “full recovery” by day 22 with a concentration below 0.25 mg/kg. Yet they found a concentration of 22.4 mg/kg in the ripe honey on day 28. In contrast, Stanimirovic et al. (2022) found a significant difference of lithium in honey taken from honey chambers of untreated colonies (0.018 mg/kg) compared to 0.034 mg/kg from lithium treated colonies, which is still far below the naturally occurring concentrations of lithium in honey.
Another study detected lithium concentrations seven days post-treatment between 0.05 and 0.9 mg/kg (Prešern et al. 2020). Our residue analysis of honey and stored bee food was conducted in the spring of the following year, which was almost ten months post treatment. We found lithium at rates of 3.1 to 5.4 mg/kg in the stored food of the brood chamber. Colonies were fed an additional 15–20 kg of sugar syrup post treatment to provide them with enough winter food stores, which must have diluted the lithium concentrations. In freshly made spring honey we harvested, we only found 0.1–0.2 mg/kg lithium which is far below the natural occurring concentrations. Our treatment method of feeding LiCl in candy does not lead to undesirable residues in honey.
LiCl provides many advantages compared to current varroacides on the market. The most frequently used non-synthetic varroacides in Middle and Northern Europe are currently formic acid in the summer and oxalic acid in the winter, both of which require specific environmental or colony conditions for high efficacy and good tolerability (Adjlane et al. 2016; Steube et al. 2021). Our new application method, unlike these organic acids, allows the beekeeper to be independent of environmental factors. Even though caging the queen, in order to create a broodless period requires additional effort and time, especially in large beekeeping operations like in the USA or Canada, such caging is becoming more standard as a method of integrated Varroa control (Büchler et al. 2020; Gregorc et al. 2017; van der Steen and Vejsnæs 2021). A brood interruption not only has the advantage that all mites are forced to reside on the adult bees, which makes them vulnerable to varroacides, it also interrupts the mite’s reproductive cycle which slows down this parasite’s population growth (Gabel et al. 2023; Jack and Ellis 2021; Rosenkranz et al. 2010). In the colonies with the repeated treatments where we allowed bees to keep rearing brood, three times as many mites fell (compare A3 with brood to A2 without brood in Table 2), even though the natural mite drop before the treatment was similar in both groups. Giacomelli et al. (2016) showed that queen caging alone resulted in a 40% reduction of Varroa populations, but should always be used in combination with a varroacide to achieve sufficient mite mortalities. As LiCl is a natural salt, the combination of queen caging and LiCl application could be a treatment option for organic beekeepers, too. A comparison of the additional workload generated by different summer brood interruption methods showed that queen caging was one of the least labor-intensive ones with no negative impact on colony strength between the different methods (Büchler et al. 2020). Rather, Lodesani et al. (2014) demonstrated that in Italy treatments that include a brood interruption approach yielded the greatest colony survival rate compared to alternative treatment methods. A broodless period during late summer allows beekeepers to remove old combs and replace them with new ones, reducing pesticide residues in the lipophilic beeswax, which has been shown to have a beneficial effect on the productivity of the colony (Berry and Delaplane 2001; Taha et al. 2021).
Conclusion
When caging the queen is integrated into a varroa treatment strategy, the application of LiCl via candy feeding represents an effective and “easy to apply” treatment due to its rapid action and even distribution within a colony, regardless of external weather conditions. The systemic mode of action makes LiCl an attractive new varroacide that deserves more attention and a communal effort to bring this new Varroa control tool to the market, where it can aid beekeepers in treating infested colonies sustainably and keeping their bees healthy.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdulkhaliq A, Swaileh KM (2017) Physico-chemical properties of multi-floral honey from the West Bank, Palestine. Int J Food Prop 20:447–454. https://doi.org/10.1080/10942912.2016.1166128
Adjlane N, Tarek E-O, Haddad N (2016) Evaluation of oxalic acid treatments against the mite varroa destructor and secondary effects on honey bees Apis mellifera. J Arthropod Borne Dis 10:501–509
Albero B, Miguel E, García-Valcárcel AI (2023) Acaricide residues in beeswax. Implications in honey, brood and honeybee. Environ Monit Assess 195:454. https://doi.org/10.1007/s10661-023-11047-6
Berry JA, Delaplane KS (2001) Effects of comb age on honey bee colony growth and brood survivorship. J Apic Res 40:3–8. https://doi.org/10.1080/00218839.2001.11101042
Berry JA, Bartlett LJ, Bruckner S, Baker C, Braman SK, Delaplane KS, Williams GR (2022) Assessing repeated oxalic acid vaporization in honey bee (Hymenoptera: Apidae) colonies for control of the ectoparasitic mite Varroa destructor. J Insect Sci 22. https://doi.org/10.1093/jisesa/ieab089
Bogdanov S, Jurendic T, Sieber R, Gallmann P (2008) Honey for nutrition and health: a review. J Am Coll Nutr 27:677–689. https://doi.org/10.1080/07315724.2008.10719745
Borsuk G, Sulborska A, Stawiarz E, Olszewski K, Wiącek D, Ramzi N, Nawrocka A, Jędryczka M (2021) Capacity of honeybees to remove heavy metals from nectar and excrete the contaminants from their bodies. Apidologie 52:1098–1111. https://doi.org/10.1007/s13592-021-00890-6
Bubnič J, Moosbeckhofer R, Prešern J, Moškrič A, Formato G, Pietropaoli M, Gregorc A, Muz MN, Škerl MIS (2021) Three pillars of Varroa control. Apidologie 52:1305–1333. https://doi.org/10.1007/s13592-021-00903-4
Büchler R, Berg S, Le Conte Y (2010) Breeding for resistance to Varroa destructor in Europe. Apidologie 41:393–408. https://doi.org/10.1051/apido/2010011
Büchler R, Uzunov A, Kovačić M, Prešern J, Pietropaoli M, Hatjina F, Pavlov B, Charistos L, Formato G, Galarza E, Gerula D, Gregorc A, Malagnini V, Meixner M, Nedić N, Puškadija Z, Rivera-Gomis J, Rogelj Jenko M, Smodiš Škerl MI, Vallon J, Vojt D, Wilde J, Nanetti A (2020) Summer brood interruption as integrated management strategy for effective Varroa control in Europe. J Apic Res 59:764–773. https://doi.org/10.1080/00218839.2020.1793278
Chantawannakul P, de Guzman LI, Li J, Williams GR (2016) Parasites, pathogens, and pests of honeybees in Asia. Apidologie 47:301–324. https://doi.org/10.1007/s13592-015-0407-5
Chapman NC, Colin T, Cook J, da Silva CRB, Gloag R, Hogendoorn K, Howard SR, Remnant EJ, Roberts JMK, Tierney SM, Wilson RS, Mikheyev AS (2023) The final frontier: ecological and evolutionary dynamics of a global parasite invasion. Biol Lett 19:20220589. https://doi.org/10.1098/rsbl.2022.0589
Conti ME, Canepari S, Finoia MG, Mele G, Astolfi ML (2018) Characterization of Italian multifloral honeys on the basis of their mineral content and some typical quality parameters. J Food Compos Anal 74:102–113. https://doi.org/10.1016/j.jfca.2018.09.002
Crailsheim K (1998) Trophallactic interactions in the adult honeybee (Apis mellifera L.). Apidologie 29:97–112. https://doi.org/10.1051/apido:19980106
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis JD (2013) Standard methods for varroa research. J Apic Res 52:1–54. https://doi.org/10.3896/IBRA.1.52.1.09
Ehrenberg S, Lewkowski O, Erler S (2019) Dyeing but not dying: Colourful dyes as a non-lethal method of food labelling for in vitro-reared honey bee (Apis mellifera) larvae. J Insect Physiol 113:1–8. https://doi.org/10.1016/j.jinsphys.2018.12.008
Elzen PJ, Westervelt D, Lucas R (2004) Formic acid treatment for control of Varroa destructor (Mesostigmata: Varroidae) and safety to Apis mellifera (Hymenoptera: Apidae) under southern United States conditions. J Econ Entomol 97:1509–1512. https://doi.org/10.1603/0022-0493-97.5.1509
European Medicines Agency (2021) Guideline on veterinary medicinal products controlling Varroa destructor parasitosis in bees (EMA/CVMP/ EWP/459883/2008-Rev.1* Committee for Medicinal Products for Veterinary Use (CVMP). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-veterinary-medicinal-products-controlling-varroa-destructor-parasitosis-bees-revision-1_en.pdf. Accessed 25 Nov 2023
Ferensztajn-Rochowiak E, Chłopocka-Woźniak M, Rybakowski JK (2021) Ultra-long-term lithium therapy: all-important matters and a case of successful 50-year lithium treatment. Braz J Psychiatry 43:407–413. https://doi.org/10.1590/1516-4446-2020-1111
Frey E, Rosenkranz P (2014) Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J Econ Entomol 107:508–515. https://doi.org/10.1603/EC13381
Frey E, Schnell H, Rosenkranz P (2011) Invasion of Varroa destructor mites into mite-free honey bee colonies under the controlled conditions of a military training area. J Apic Res 50:138–144. https://doi.org/10.3896/IBRA.1.50.2.05
Fuchs S (1985) Untersuchungen zur quantitativen Abschätzung des Befalls von Bienenvölkern mit Varroa jacobsoni Oud. und zur Verteilung des Parasiten im Bienenvolk. Apidologie 16:343–368. https://doi.org/10.1051/apido:19850401
Fuchs S (1992) Choice in Varroa jacobsoni Oud. between honey bee drone or workerbrood cells for reproduction. Behav Ecol Sociobiol 31:429–435. https://doi.org/10.1007/BF00170610
Gabel M, Scheiner R, Büchler R (2023) Immediate and long-term effects of induced brood interruptions on the reproductive success of Varroa destructor. Apidologie 54:1–17. https://doi.org/10.1007/s13592-023-00998-x
Genersch E, von der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R, Berg S, Ritter W, Mühlen W, Gisder S, Meixner M, Liebig G, Rosenkranz P (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41:332–352. https://doi.org/10.1051/apido/2010014
Giacobino A, Miotti C, Molineri A, Orellano E, Signorini M, Pacini A (2023) Short communication: Varroa destructor re-invasion dynamics during autumn and winter in Apis mellifera colonies from a temperate climate. J Invertebr Pathol 197:107890. https://doi.org/10.1016/j.jip.2023.107890
Giacomelli A, Pietropaoli M, Carvelli A, Iacoponi F, Formato G (2016) Combination of thymol treatment (Apiguard®) and caging the queen technique to fight Varroa destructor. Apidologie 47:606–616. https://doi.org/10.1007/s13592-015-0408-4
Gomes-da-Costa S, Marx W, Corponi F, Anmella G, Murru A, Pons-Cabrera MT, Giménez-Palomo A, Gutiérrez-Arango F, Llach CD, Fico G, Kotzalidis GD, Verdolini N, Valentí M, Berk M, Vieta E, Pacchiarotti I (2022) Lithium therapy and weight change in people with bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev 134:104266. https://doi.org/10.1016/j.neubiorev.2021.07.011
Gray A, Brodschneider R, Adjlane N, Ballis A, Brusbardis V, Charrière J-D, Chlebo R, Coffey FM, Cornelissen B, Da Amaro Costa C, Csáki T, Dahle B, Danihlík J, Dražić MM, Evans G, Fedoriak M, Forsythe I, Graaf de D, Gregorc A, Johannesen J, Kauko L, Kristiansen P, Martikkala M, Martín-Hernández R, Medina-Flores CA, Mutinelli F, Patalano S, Petrov P, Raudmets A, Ryzhikov VA, Simon-Delso N, Stevanovic J, Topolska G, Uzunov A, Vejsnaes F, Williams A, Zammit-Mangion M, Soroker V (2019) Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J Apic Res 58:479–485. https://doi.org/10.1080/00218839.2019.1615661
Gregorc A, Alburaki M, Werle C, Knight PR, Adamczyk J (2017) Brood removal or queen caging combined with oxalic acid treatment to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera). Apidologie 48:821–832. https://doi.org/10.1007/s13592-017-0526-2
Haarmann T, Spivak M, Weaver D, Weaver B, Glenn T (2002) Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J Econ Entomol 95:28–35. https://doi.org/10.1603/0022-0493-95.1.28
Hernandez J, Hattendorf J, Aebi A, Dietemann V (2022) Compliance with recommended Varroa destructor treatment regimens improves the survival of honey bee colonies over winter. Res Vet Sci 144:1–10. https://doi.org/10.1016/j.rvsc.2021.12.025
Higes M, Martín-Hernández R, Hernández-Rodríguez CS, González-Cabrera J (2020) Assessing the resistance to acaricides in Varroa destructor from several Spanish locations. Parasitol Res 119:3595–3601. https://doi.org/10.1007/s00436-020-06879-x
Ifantids MD (1988) Some aspects of the process of Varroa jacobsoni mite entrance into honey bee (Apis mellifera) bood cells. Apidologie 19:387–396. https://doi.org/10.1051/apido:19880406
Jack CJ, Ellis JD (2021) Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) colonies. J Insect Sci 21. https://doi.org/10.1093/jisesa/ieab058
Jovanovic NM, Glavinic U, Ristanic M, Vejnovic B, Stevanovic J, Cosic M, Stanimirovic Z (2022) Contact varroacidal efficacy of lithium citrate and its influence on viral loads, immune parameters and oxidative stress of honey bees in a field experiment. Front Physiol 13:1000944. https://doi.org/10.3389/fphys.2022.1000944
Karabagias IK, Louppis AP, Karabournioti S, Kontakos S, Papastephanou C, Kontominas MG (2017) Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem 217:445–455. https://doi.org/10.1016/j.foodchem.2016.08.124
Kast C, Kilchenmann V, Charrière J-D (2021) Long-term monitoring of lipophilic acaricide residues in commercial Swiss beeswax. Pest Manag Sci 77:4026–4033. https://doi.org/10.1002/ps.6427
Kolics É, Mátyás K, Taller J, Specziár A, Kolics B (2020) Contact effect contribution to the high efficiency of lithium chloride against the mite parasite of the honey bee. Insects 11:333. https://doi.org/10.3390/insects11060333
Kolics É, Specziár A, Taller J, Mátyás KK, Kolics B (2021b) Lithium chloride outperformed oxalic acid sublimation in a preliminary experiment for Varroa mite control in pre-wintering honey bee colonies. Acta Vet Hung 68:370–373. https://doi.org/10.1556/004.2020.00060
Kolics B, Kolics É, Mátyás K, Taller J, Specziár A (2022) Comparison of alternative application methods for anti-Varroa lithium chloride treatments. Insects 13:633. https://doi.org/10.3390/insects13070633
Kolics É, Sajtos Z, Mátyás K, Szepesi K, Solti I, Németh G, Taller J, Baranyai E, Specziár A, Kolics B (2021a) Changes in lithium levels in bees and their products following anti-varroa treatment. Insects 12. https://doi.org/10.3390/insects12070579
LeBoeuf AC (2017) Trophallaxis. Curr Biol 27:R1299–R1300. https://doi.org/10.1016/j.cub.2017.10.047
Lodesani M, Costa C, Besana A, Dall’Olio R, Franceschetti S, Tesoriero D, Giacomo D (2014) Impact of control strategies for Varroa destructor on colony survival and health in northern and central regions of Italy. J Apic Res 53:155–164. https://doi.org/10.3896/IBRA.1.53.1.17
Mitton GA, Meroi Arcerito F, Cooley H, Fernández de Landa G, Eguaras MJ, Ruffinengo SR, Maggi MD (2022) More than sixty years living with Varroa destructor : a review of acaricide resistance. Int J Pest Manag:1–18. https://doi.org/10.1080/09670874.2022.2094489
Mondet F, Parejo M, Meixner MD, Costa C, Kryger P, Andonov S, Servin B, Basso B, Bieńkowska M, Bigio G, Căuia E, Cebotari V, Dahle B, Dražić MM, Hatjina F, Kovačić M, Kretavicius J, Lima AS, Panasiuk B, Pinto MA, Uzunov A, Wilde J, Büchler R (2020) Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.). Insects 11:595. https://doi.org/10.3390/insects11090595
Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y (2020b) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int J Parasitol 50:433–447. https://doi.org/10.1016/j.ijpara.2020.03.005
Mutinelli F (2016) Veterinary medicinal products to control Varroa destructor in honey bee colonies (Apis mellifera ) and related EU legislation – an update. J Apic Res 55:78–88. https://doi.org/10.1080/00218839.2016.1172694
Nixon HL, Ribbands CR (1952) Food transmission within the honeybee community. Proc R Soc Lond B Biol Sci 140:43–50. https://doi.org/10.1098/rspb.1952.0042
Park W (1925) The Storing and Ripening of Honey by Honeybees. J Econ Entomol 18:405–410. https://doi.org/10.1093/jee/18.2.405
Pietropaoli M, Formato G (2019) Acaricide efficacy and honey bee toxicity of three new formic acid-based products to control Varroa destructor. J Apic Res 58:824–830. https://doi.org/10.1080/00218839.2019.1656788
Prešern J, Kur U, Bubnič J, Šala M (2020) Lithium contamination of honeybee products and its accumulation in brood as a consequence of anti-varroa treatment. Food Chem 330:127334. https://doi.org/10.1016/j.foodchem.2020.127334
Qadir ZA, Idrees A, Mahmood R, Sarwar G, Bakar MA, Ahmad S, Raza MM, Li J (2021) Effectiveness of different soft acaricides against honey bee ectoparasitic Mite Varroa destructor (Acari: Varroidae). Insects 12:1032. https://doi.org/10.3390/insects12111032
Rademacher E, Harz M, Schneider S (2017) Effects of Oxalic Acid on Apis mellifera (Hymenoptera: Apidae). Insects 8:84. https://doi.org/10.3390/insects8030084
Rein C, Makosch M, Renz J, Rosenkranz P (2022) Lithium chloride leads to concentration dependent brood damages in honey bee hives (Apis mellifera) during control of the mite Varroa destructor. Apidologie 53:1–14. https://doi.org/10.1007/s13592-022-00949-y
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):96–119. https://doi.org/10.1016/j.jip.2009.07.016
Satta A, Floris I, Eguaras M, Cabras P, Garau VL, Melis M (2005) Formic acid-based treatments for control of Varroa destructor in a Mediterranean area. J Econ Entomol 98:267–273. https://doi.org/10.1093/jee/98.2.267
Schur A, Tornier I, Brasse D, Muhlen W, von der Ohe W, Wallner K, Wehling M (2003) Honey bee brood ring-test in 2002: method for the assessment of side effects of plant protection products on the honey bee brood under semi-field conditions. Bull Insectol 56:91–96
Seidel U, Baumhof E, Hägele FA, Bosy-Westphal A, Birringer M, Rimbach G (2019) Lithium-rich mineral water is a highly bioavailable lithium source for human consumption. Mol Nutr Food Res 63:e1900039. https://doi.org/10.1002/mnfr.201900039
Seidel U, Jans K, Hommen N, Ipharraguerre IR, Lüersen K, Birringer M, Rimbach G (2020) Lithium content of 160 beverages and its impact on lithium status in Drosophila melanogaster. Foods 9. https://doi.org/10.3390/foods9060795
Seitz N, Traynor KS, Steinhauer N, Rennich K, Wilson ME, Ellis JD, Rose R, Tarpy DR, Sagili RR, Caron DM, Delaplane KS, Rangel J, Lee K, Baylis K, Wilkes JT, Skinner JA, Pettis JS, vanEngelsdorp D (2015) A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J Apic Res 54:292–304. https://doi.org/10.1080/00218839.2016.1153294
Semkiw P, Skubida P, Pohorecka K (2013) The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. Journal of Apicultural Science 57:107–121. https://doi.org/10.2478/jas-2013-0012
Stahlmann-Brown P, Hall RJ, Pragert H, Robertson T (2022) Varroa appears to drive persistent increases in New Zealand colony losses. Insects 13. https://doi.org/10.3390/insects13070589
Stanimirovic Z, Glavinic U, Jovanovic NM, Ristanic M, Milojković-Opsenica D, Mutic J, Stevanovic J (2022) Preliminary trials on effects of lithium salts on Varroa destructor, honey and wax matrices. J Apic Res 61:375–391. https://doi.org/10.1080/00218839.2021.1988277
Steube X, Beinert P, Kirchner WH (2021) Efficacy and temperature dependence of 60% and 85% formic acid treatment against Varroa destructor. Apidologie 52:720–729. https://doi.org/10.1007/s13592-021-00859-5
Szklarska D, Rzymski P (2019) Is lithium a micronutrient? From biological activity and epidemiological observation to food fortification. Biol Trace Elem Res 189:18–27. https://doi.org/10.1007/s12011-018-1455-2
Taha E-KA, Rakha OM, Elnabawy E-SM, Hassan MM, Shawer DM (2021) Comb age significantly influences the productivity of the honeybee (Apis mellifera) colony. Journal of King Saud University - Science 33:101436. https://doi.org/10.1016/j.jksus.2021.101436
Tariba Lovaković B, Lazarus M, Brčić Karačonji I, Jurica K, Živković Semren T, Lušić D, Brajenović N, Pelaić Z, Pizent A (2018) Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J Trace Elem Med Biol 45:85–92. https://doi.org/10.1016/j.jtemb.2017.09.022
Travis DA, Watson RP, Tauer A (2011) The spread of pathogens through trade in wildlife. Revue Scientifique Et Technique-OIE 30:219
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36:592–606. https://doi.org/10.1016/j.pt.2020.04.004
Underwood RM, Currie RW (2003) The effects of temperature and dose of formic acid on treatment efficacy against Varroa destructor (Acari: Varroidae), a parasite of Apis mellifera (Hymenoptera: Apidae). Exp Appl Acarol 29:303–313. https://doi.org/10.1023/A:1025892906393
van der Steen J, Vejsnæs F (2021) Varroa control: a brief overview of available methods. Bee World 98:50–56. https://doi.org/10.1080/0005772X.2021.1896196
Vilarem C, Piou V, Vogelweith F, Vétillard A (2021) Varroa destructor from the laboratory to the field: control, biocontrol and IPM perspectives-a review. Insects 12:800. https://doi.org/10.3390/insects12090800
Ziegelmann B, Abele E, Hannus S, Beitzinger M, Berg S, Rosenkranz P (2018) Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci Rep 8:683. https://doi.org/10.1038/s41598-017-19137-5
Acknowledgements
Our thanks are due to Dr. Sandra Renz and Andrea Ruf from the Core Facility Hohenheim for the analysis of lithium in samples. We also express our gratitude to Julia Renz and Markus Grünke for dissection of the bee samples in 2021. We appreciate the help of Nick Baumann and Adrian Preusch with the experiments in 2022, including colony management and sampling.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (Grant number 281C301A19).
Author information
Authors and Affiliations
Contributions
CR: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualization, project administration, funding acquisition. MB: conceptualization, methodology, investigation. KT: writing—review and editing. PR: conceptualization, methodology, validation, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Section Editor: Van Lun Low
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rein, C., Blumenschein, M., Traynor, K. et al. Lithium chloride treatments in free flying honey bee colonies: efficacy, brood survival, and within-colony distribution. Parasitol Res 123, 67 (2024). https://doi.org/10.1007/s00436-023-08084-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08084-y