Abstract
A 2-year field experiment was performed to test lithium chloride, LiCl, application in a normal beekeeping management system. The effect of LiCl on bee larval mortality, beehive weight (honey production) and Varroa mite mortality were tested. Spectrometric quantification of Li on honey and the larval body were made to test the effectiveness of the presence of LiCl. Li was detected in bee larval bodies and in honey over 2 years, from 2018 to 2019. According to the results, no effect of LiCl on mite mortality or bee larval mortality was detected in the first year of application. By assessing the weight variation of beehives, only one LiCl-treated hive showed a significantly higher weight, whereas no other differences were detected between treatments and control. The same trend seen in 2018 was repeated in 2019, while a total bee larval mortality was observed after the first LiCl application, and still no differences in Varroa mite mortality were observed. According to these results, it was concluded that LiCl has no effect on Varroa mite mortality during normal beekeeping practice; furthermore, the recommended amount of treatment (25 mM) had a lethal effect (i.e., total mortality) on larvae following repeated applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey bees and their products play a central role in agriculture and the food industry worldwide. However, severe colony losses have been widely detected, especially in the USA and Europe (Hung et al. 2018). Such high colony losses do not only influence hive management but also increase the costs of pollination services (Calderone 2012; Ziegelmann et al. 2018). One of the most problematic pests of honeybee is the haemolymph-sucking ectoparasitic mite Varroa destructor (Acari: Varroidae), which originally parasitized the Eastern honey bee Apis cerana (Rosenkranz et al. 2010; Neumann and Carreck 2010; Conte et al. 2010). However, during the past 70 years, Varroa mite infestation in Apis mellifera has increased significantly and becomes a global problem, posing one of the greatest threats to modern apiculture (Rosenkranz et al. 2010; Martin et al. 2020).
Chemical control against Varroa mites can be both effective and low risk in terms of selecting for resistance. However, there are severe restrictions in some countries to use certain chemicals (Milani 2001; Maggi et al. 2016). Accordingly, the success and viability of the global apicultural industry is based on the use of only a few compounds for the control of Varroosis, i.e., the organophosphate coumaphos, some pyrethroids and the formamidine amitraz (Ziegelmann et al. 2018). As a direct result of the low number of chemicals available for use against Varroa mites, increased resistance to all available synthetic acaricides has occurred (Maggi et al. 2011; Kamler et al. 2016). Therefore, new methods of controlling the mite are of significant interest, and some products such as lithium chloride, LiCl, have already been suggested as a potential solution for combatting Varroa populations under controlled lab conditions [3]: according to this particular study, 100% mite mortality was observed in the brood-free period with little or no mortality of adult bees. Besides this study, only a very few other studies have used LiCl to test the effective of this ionic compound as a contact treatment against Varroa mites (Kolics et al. 2020), and none of these recorded its effectiveness under normal beekeeping management, i.e., the way that may be practically important for beekeepers. Moreover, there is evidence that lithium acts on the human nervous system (Nonaka et al. 1998; Chang et al. 1999), where it has proved to be benefical in the treatment of bipolar disorder by decreasing depression and suicide.
During the present study, we hypothesized and subsequently empirically tested the possibility that application of LiCl during normal beekeeping management might have a detrimental effect on larval mortality and hence prove a ‘two-edged sword’ in terms of combatting the mite. It is clearly important to demonstrate whether the salt is indeed a viable and safe agent against the mite, not only in terms of efficiacy againt the pest but also in terms of bee health and that of humans and other animals imbibing the honey from bee colonies thus treated.
Material and methods
A 2-year field experiment was performed to test this hypothesis. The bees and parasitizing mites treated with LiCl were examined at the beginning of the summer of 2018 under normal beekeeping practice in Langstroth beehives. Before the experiment started, 10 beehives were selected and paired after weight. The test hives were subjected to the LiCl treatment. In these treated hives, a feeder was provided for the bees containing sugar syrup (cane sugar at a 1:1 concentration of sugar and water by volume) mixed with crystalline lithium chloride to give a final total concentration of 25 mM LiCl. In the case of the control hives, the same feeding solution, but lacking LiCl, was administered to the bees. The treatments were performed five times at an interval of four days. This was done in order to cover a 21-day larval development cycle, so that all larvae were fed at least once with the sugar syrup containing LiCl during their development. The control colonies received 2 dl of sugar syrup, while the treated ones received 2 dl of LiCl in the same concentration of sugar syrup. No other treatments were performed during the experiment.
After feeding the bees with the sugar syrup mixed plus LiCl, samples were taken from pupa and larvae daily (for 3 days) and subsequently every 2–3 days for 3 weeks. Thereafter, the sampled larvae were frozen immediately. After three weeks of treatment, honey and wax were also sampled. Again, the samples were immediately frozen, but here subsequently examined by atomic absorption spectroscopy for the presence or absence of Li. After starting the treatment, high resolution photographs of the developing larvae were taken daily (for 3 days) and later for 2–3 days over a period of 3 weeks. In the photographs, 100–100 larvae from both sides of the brood frame were randomly selected for each colony. Young 3–4-day-old larvae were selected and examined to see if the individuals reached the pupal stage within the given time period or rather, died. After pupation, we further examined whether the individuals developed within the specified interval. If the covered cells became empty sooner than the honey bee was supposed to have developed this meant that the individual had died during development and the bees had cleared the worker brood cell. At the same time, by examining all photographs we were able to assess whether there was a delay in the development of larvae as a result of LiCl treatment compared with control colonies. The weight of each beehive was assessed every day during the experiment by simple measuring in kg the beehive mass using a libra. Mite mortality was also assessed by collecting 100 individuals from each beehive and living or dead exemplars recorded for both treatment and control.
The experiment was repeated again in 2019. In this second year, 20 colonies were selected with the same population from the apiary. These colonies comprised the same ten (five LiCl-treated and five control) which were chosen in 2018, plus ten new colonies. The colonies had much higher numbers of worker bees than those from the previous year. To assess the possible effect of LiCl, the 10 treated colonies received 6 dl of sugar syrup with LiCl, whilst the 10 control colonies received the same amount of simple sugar syrup. LiCl concentration was the same, i.e., 25 mM. Additional treatments were also performed every four days and five times in total. This was done one week after the LiCl treatments, so that the colonies (both control and LiCl) were treated with Varachet (Amitrase 16% and Taufluvalina 6%). Before treatment, a white sheet of paper was placed under varroa mesh floor of each hive. After treatment, the white sheets were removed and mites counted.
Sample preparation for LiCl analyses
The samples were chemically processed before analysis. The solutions used for the digestion contained: 65% HNO3 (Merck, suprapure) and 30% H2O2 (Chempur, pure). All digests were diluted to a final volume of 50 mL with ultrapure water (Millipore Direct-Q, 3 UV System, Merck, Germany). The PTFE vessels and the beakers were previously decontaminated with HNO3:H2O = 10:1 washing solution for 24 h, then rinsed with ultrapure water. The bee larvae samples, each consisting of ten specimens from the same hive, were dried at 105 °C, and then weighed on an analytical balance (OHAUS Europe GmbH, Switzerland). The sample mass varied between ~ 100 and 200 mg. Prior to the digestion procedure, the wax samples were liquefied by heating to 70 °C in a water bath, the liquids were immediately homogenized with a stirrer at 700 rpm, until they solidified. Aliquots of 0.22–0.26 g were weighed in PTFE vessels. Both larvae and wax samples were digested at 170 °C in acidic-oxidative medium (6 mL 65% HNO3 and 2 mL 30% H2O2) for 15 min. using a microwave digestion system (speedwave ENTRY, Berghof Group, Germany). Prior to digestion, the honey samples were liquefied by heating at 60 °C in water bath, and homogenized by stirring with a glass rod. Aliquots of 0.20–0.27 g were weighed and placed in 25 mL glass beakers. The digestion was performed at atmospheric pressure, in a solution of 5 mL 65% HNO3: 2 mL 30% H2O2.
Spectrometric quantification of LiCl
The lithium ion was quantified by microwave plasma atomic emission spectrometry (MP-AES) technique. This technique is one of the most suitable ones for such analysis, as it has high sensibility for this element. The wavelength of the Li used in the measurements was the atomic line of 670.8 nm, with a signal integration time s of 3 s and sample introduction speed of 15 rpm. We achieved a method detection limit of 0.01 mg kg−1 for the larvae (dry matter) and 0.06 mg kg−1 for the honey and wax (fresh weight). Analyses were performed with an MP-AES 4210 spectrometer (Agilent Technologies, USA). Cesium-chloride (CsCl) was used as ionization suppressor and matrix modifier in a final concentration of 1 g L−1 for Cs. We validated the method with a certified reference material (TMDA-70.2, high level fortified water sample for trace elements). Three subsamples were prepared containing the same CsCl concentration as the samples. The recovery was of 98.1% ± 2.8%.
Data analyses
For LiCl detection and estimation of mite mortality in 2018, data were compared using the Kruskal–Wallis followed by Mann–Whitney U tests, as neither data sets were assumed to be normally distributed. Bee larvae mortality assessment from pictures were compared using a paired t test. Data of weight (kg) variation of treated and control beehives in 2018 were log10 transformed, and analyzed. As the data were normally distributed, ANOVA followed by Tukey tests were run, and each set of replicated assessment were used as variables. Treatments and controls for the 2019 data sets were similarly compared, here only data from 9 treatments and control hives were used, an one from each were destroyed during the experiment. Varroa mite mortality data was also compared using ANOVA followed by Tukey testing. All data analyses were performed in PAST version 4.0.
Results
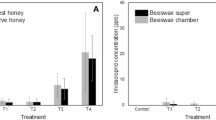
As expected, LiCl was seen to be present at a significantly high level in larval body tissues when beehives were treated with this compound. However, on comparing the effect of LiCl on mite mortality, no differences between LiCl treatments and control were detected (Fig. 1).
Furthermore, no effects of LiCl were detected when photographs were analyzed and the larval and mite mortality assessed. Larval mortality was found to be relatively similar between the controls and treatments (DF = 8, t = 0.2, p < 0.8) as it was in terms of mite mortality (DF = 99, t = 0.4, p < 0.57) (Fig. 2).
By assessing the weight variation of beehives, only one LiCl-treated hive showed a significantly higher weight, although no other differences were detected between treatments and control (Fig. 3).
The presence of LiCl was detected in bee larval body tissues both in 2018 and 2019. No differences in larval mortality (DF = 99, F = 1.2, p < 0.09), hives weight (DF = 8, F = 0.56, p < 0.45) and Varroa mortality (DF = 99, F = 0.2, p < 0.76) were found when hives used in 2018, and re-used in 2019, were compared with newly used hives in 2019. For the 2019 data, no differences in mite mortality were detected between LiCl and control plots, while some variations in mortality were observed (Fig. 4).
We were not able to perform statistical analyses on the 2019 data larval mortality because all LiCl treated bee larvae died after the first LiCl application (100% dead rate for those used for the second year and 80% for those used from 2018).
Discussion
The present study reveals that when first applied, the LiCl treatment as used (25 mM) had no significant effect on bee larval mortality during normal beekeeping practice, whereas total mortality resulted from repeated applications at such concentrations (i.e., in the second year according to our results). In addition, no effect on bee weight was detected, revealing that all workers apparently maintained normal metabolic activity following LiCl applications. Previously published research has shown a 3–7% mortality of caged bees after similar LiCl treatment, while the effect of LiCl on Varroa mites was significant, and as a result, use of the compound was recommended as a potential miticidal agent (Ziegelmann et al. 2018).
According to our results, LiCl had no significant effect on Varroa mite mortality under normal beekeeping conditions; moreover, the recommended treatment of 25 mM caused a total mortality of honey bee larvae after repeated applications. One explanation of the negative effect of such LiCl application may be due to the variation in bee activity under normal beekeeping practice, such that high movement of workers may neutralize the negative effects of LiCl application, whereas after a few days, especially when the workers are not so active (for example on rainy days), the accumulated toxic effects of LiCl on larval development as a result of continued feeding by the worker bee adults finally become apparent.
Other similar studies have also reported that the lowest tested concentration (10.8 mM) of LiCl solution was highly effective against Varroa mites in the contact mode of action (Kolics et al. 2020). While there is evidence that Li+ ions have effects on the human nervous system (Chang et al. 1999), more especially as commonly used in the treatment of manic depressive illness (Nonaka et al. 1998). Thus, its application in normal beekeeping practice, where bee products are used and more especially since LiCl accumulation in honey has earlier been demonstrated (Ziegelmann et al. 2018), means that its deleterious effect on humans, notably on children, must be seriously considered. The total bee larval mortality, as here shown, indicates that LiCl should not be used for the control of Varroa mites during normal beekeeping practice and involving the supply of honey to humans, their pets, livestock and indeed to wildlife generally, e.g., hummingbirds in the Americas.
Conclusion on future biology
According to the results, LiCl effects have to be tested further under normal beekeeping management. It has to be mentioned that the long-term effects of LiCl are not known for individual hives. In addition, is important to know if LiCl is incorporated into the beeswax, or it accumulates in bee and larval body too, and what kind of long-term change it causes in comparison with other treatments.
One important assessment would be to compare how and why differences in Varroa mites’ mortality occurs when LiCl is applied by spraying in comparison with other applications. Altogether, such highly important and economically significant treatments needs to be tested under different beekeeping management systems and factors such as environmental effects, bee activity, LiCl applications and specific effects on individual hives must be considered.
Data availability
No restrictions on data availability were formulated, and all data were collected from public sources using personally designed software. The text, illustrations, and any other materials included in the manuscript do not infringe any existing copyright or other rights of anyone.
References
Calderone NW (2012) Insect pollinated crops, insect pollinators and us agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7:e37235. https://doi.org/10.1371/journal.pone.0037235
Chang MCJ, Bell JM, Purdon AD et al (1999) Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem Res 24:399–406. https://doi.org/10.1023/A:1020989701330
Conte YL, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41:353–363. https://doi.org/10.1051/apido/2010017
Hung K-LJ, Kingston JM, Albrecht M et al (2018) The worldwide importance of honey bees as pollinators in natural habitats. Proc R Soc b: Biol Sci 285:20172140. https://doi.org/10.1098/rspb.2017.2140
Kamler M, Nesvorna M, Stara J et al (2016) Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp Appl Acarol 69:1–9. https://doi.org/10.1007/s10493-016-0023-8
Kolics É, Mátyás K, Taller J et al (2020) Contact effect contribution to the high efficiency of lithium chloride against the mite parasite of the honey bee. Insects 11:333. https://doi.org/10.3390/insects11060333
Maggi M, Tourn E, Negri P et al (2016) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47:596–605. https://doi.org/10.1007/s13592-015-0405-7
Maggi MD, Ruffinengo SR, Mendoza Y et al (2011) Susceptibility of Varroa destructor (Acari: Varroidae) to synthetic acaricides in Uruguay: Varroa mites’ potential to develop acaricide resistance. Parasitol Res 108:815–821. https://doi.org/10.1007/s00436-010-2122-5
Martin SJ, Hawkins GP, Brettell LE et al (2020) Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 51:369–381. https://doi.org/10.1007/s13592-019-00721-9
Milani N (2001) Activity of oxalic and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 32:127–138. https://doi.org/10.1051/apido:2001118
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apic Res 49:1–6. https://doi.org/10.3896/IBRA.1.49.1.01
Nonaka S, Hough CJ, Chuang D-M (1998) Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-d-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A 95:2642–2647
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):S96-119. https://doi.org/10.1016/j.jip.2009.07.016
Ziegelmann B, Abele E, Hannus S et al (2018) Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci Rep 8:683. https://doi.org/10.1038/s41598-017-19137-5
Acknowledgements
This article was founded by the Institute of Research Programs of the Sapientia Hungarian University of Transylvania. We are grateful to Prof. Hugh D. Loxdale for his professional comments and English review.
Funding
Open access funding provided by Sapientia Hungarian University of Transylvania. This research received no external funding.
Author information
Authors and Affiliations
Contributions
ID and AB conceived the experiments. ID, LK and MS designed and performed the experiments. ARZ made the chemical analyses. ID, MS, LK and AB analyzed the data. AB, LK and ID wrote the manuscript. AB, ID, MS, LK and ARZ provided editorial advice.
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this article have no financial or other conflict of interest to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demeter, I., Sárospataki, M., Zsigmond, A.R. et al. Deleterious effect of LiCl on honeybee (Aphis mellifera) grubs and no effect on Varroa mites (Varroa destructor) under normal beekeeping management. BIOLOGIA FUTURA 75, 199–204 (2024). https://doi.org/10.1007/s42977-023-00196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-023-00196-x