Abstract
The parasitic mite Varroa destructor (Anderson & Trueman) spends the dispersal phase of its life cycle on adult honeybees (Apis mellifera L.). The meaning of this phase for both bees and mites is still not well understood. This especially applies to prolonged dispersal phases as a result of brood interruptions. Hence, it is highly important to unravel this phase for understanding the underlying biological mechanisms and implementing this knowledge in beekeeping practice and research efforts. We investigated the effects of brood interruptions on honeybee colonies and the mites naturally infesting them. Reproduction parameters, brood infestation and recapping frequency were monitored over 60 days after brood interruptions of varying durations. Our results show that recapping frequency and mite non-reproduction increased during the interruption of egg laying. The duration of interruption and the time elapsed afterwards additionally affected the occurrence of reproductive failure. Hence, the reproduction of mites was affected by brood breaks immediately and in the long run.
Similar content being viewed by others
1 Introduction
The ectoparasitic bee mite Varroa destructor is the major pathological threat for Western honeybees (Apis mellifera) and apiculture (Dietemann et al. 2012, 2013; Nazzi and Le Conte 2015; Rosenkranz et al. 2010; Vanengelsdorp et al. 2009). Many aspects of the delicate host-parasite relationship are well understood, since they have been studied intensively for decades (Nazzi and Le Conte 2015; Rosenkranz et al. 2010). However, large parts of the biology of the mite remain unclear. One example is the complex mating and reproduction biology of the mite, which plays a crucial role in population development and long-term colony survival (Fries and Rosenkranz 1996; Le Conte et al. 2020; Locke 2016; Otten 1991). The mite’s life cycle comprises two phases: (1) a reproductive phase inside the brood cells and (2) a dispersal phase (often called “phoretic” in a broader sense) on adult honeybees (Traynor et al. 2020; Nazzi and Le Conte 2015; Rosenkranz et al. 2010). Both phases seem to be affected by various factors, which can lead to a suppressed reproductive success of the mites (Grindrod and Martin 2021; Locke 2016; Mondet et al. 2020a, b). The reproductive phase and invasion of brood cells have been studied intensively, giving insights into factors like brood type (Boot et al. 1992, 1995a, b; Fuchs 1990), olfactory cues (Frey et al. 2013; Garrido and Rosenkranz 2003; Rosenkranz and Garrido 2004), hygienic behaviour (Harris 2007; Mondet et al. 2016; Mondet et al. 2020a, b), intraspecific competition (Donzé et al. 1996; Donzé and Guerin 1994; Martin 1995b; Nazzi and Milani 1996) and duration of the post-capping period of brood cells (Büchler et al. 2010; Mondet et al. 2020a, b), which modulate the reproductive success of the mites. The dispersal phase has been studied less intensively, because mites are difficult to follow on adult bees at colony level (Fries and Rosenkranz 1996). Nevertheless, the dispersal phase between consecutive reproductive attempts likely plays a crucial role for the survival and reproduction of the parasite. Especially prolonged durations caused by brood breaks might affect the following reproductive phase.
Since researchers and breeders aim for comparable data by using mites of similar physiological and reproductive states, possible effects of previous brood breaks should be considered. For example, a lower reproductive success of mites was frequently reported for naturally surviving colonies (Grindrod and Martin 2021; Locke 2016; Oddie et al. 2018). Thus, this phenomenon is regarded as a selection criterion for breeding towards Varroa resistance (Büchler et al. 2010, 2020a, b; Mondet et al. 2020b), often measured after artificial infestation with mites gained from broodless donor colonies. Hence, such measurements on colony level might be distorted, if the expression of reproductive failure per se would be altered by brood interruptions. On the other hand, the same effects might be of special interest for beekeepers, particularly if the reproductive success of mites can be decreased. Though beneficial effects of swarm-related brood breaks on mite infestation of untreated colonies are known (Loftus et al. 2016; Seeley and Smith 2015; Fries et al. 2003), the infestation levels seem to be affected by multiple factors (Fries et al. 2003). Thus, the implementation of such brood breaks in practical beekeeping is usually combined with acaricide treatments (Büchler et al. 2020b).
Studies on the dispersal part of the life cycle of mites have so far mainly focused on host preferences in terms of age and task of the adult bees parasitized (Cervo et al. 2014; Xie et al. 2016) or invasion behaviour (Beetsma et al. 1999). Though host preference may change with infestation on colony level (Cervo et al. 2014), mites prefer nurse bees as adult hosts over foragers and freshly emerged bees. This preference also corresponds to a better reproductive success of mites previously parasitizing nurse bees as adult hosts (Xie et al. 2016). Likewise, Stürmer and Rosenkranz (1994) reported a higher reproductive success of mites formerly parasitizing in colonies containing nurse bees (i.e. colonies with open brood) in comparison to mites spending their dispersal phase in colonies without brood and nurse bees. The reproductive success of these mites was decreased after artificially prolonged dispersal phases of up to 12 weeks in broodless colonies (Stürmer and Rosenkranz 1994). While no effect of the duration of naturally chosen dispersal phases was reported (Boot et al. 1995a, b; Piou et al. 2016), these findings indicate that the reproductive success of mites can be artificially altered depending on the duration of the previous dispersal phase. Such a possible effect of brood interruption is crucial for (1) bee breeding and (2) science in which mites of comparable states are needed for various bioassays respectively (Dietemann et al. 2013), as well as (3) practical beekeeping in which brood interruption methods are valued for Varroa control (Büchler et al. 2020b).
Hence, we here investigated immediate and long-term effects of induced brood interruptions of different durations on the reproductive success of Varroa destructor on colony level.
2 Materials and methods
Experiments were conducted in the summer of 2019 at the LLH Bee Institute Kirchhain (Hesse, Germany) with 27 full-grown colonies headed by open mated queens derived from different mothers of the Institute’s Carniolan breeding stock. All colonies were lodged in hives comparable to two Langstroth standard boxes and placed at the same apiary, while replicates belonging to the respective experimental groups were distributed randomly over the location. Queens were either caged in mid-July for 10, 20, or 30 days (n = 7 each) to induce an interruption of egg laying of corresponding duration or were left uncaged as a control (n = 6). For queen caging, standard cages with queen excluder sidewalls were used as described by Büchler et al. (2020b).
2.1 Sampling
Brood combs of treatment groups were subsequently sampled for brood investigation at four time points: (1) while queens were caged (10 days after caging), (2) in the first supposed brood cycle of mites after caging, (3) in the second supposed brood cycle of mites after caging and (4) in the third supposed brood cycle of mites after caging (Figure 1). Thus, the first set of brood combs (1) was sampled at the same date in all treatment groups (Figure 1). The following three sampling dates after the release of the queens (2nd, 3rd and 4th sets of brood combs) differed according to the duration of caging in the respective groups (Figure 1). Irrespective of the date, the subsequent brood combs were sampled in 20 days intervals (i.e., 2nd, 20 days; 3rd, 40 days; and 4th, 60 days) after the release date of the queens respectively (Figure 1). This timing enabled investigations on the reproductive success of mites according to Büchler et al. (2017), since most mites perform a dispersal phase of approximately 7 days before invading a cell in the L5 larval stage (Boot et al. 1993; Harbo and Harris 1999; Rosenkranz et al. 2010; Sammataro et al. 2000) and thus were found in a suitable brood age for investigation (i.e., 7–12 days post capping) of reproductive parameters after 20 days. Though the date of cell-invasion can only be extrapolated due to variation in individual mites’ behaviour, the time frame of 7 days post capping up to emergence of the bee allows for some flexibility in the investigation of reproductive success (Büchler et al. 2017). Additionally, brood combs of untreated control colonies with constant brood rearing activity were sampled. This sampling was performed four times to account for possible seasonal variation (Otten 1991) while avoiding an oversampling (i.e., weakening) of control colonies (Figure 1). These control samples were distributed over the course of the study to keep the time span between samplings as short as possible (Figure 1), since differences between long-term measurements of mite reproduction were found to be higher than in measurements in quick succession (Eynard et al. 2020).
Schematic overview over sampling dates of brood combs. Queens were caged for 10, 20, or 30 days to induce a brood interruption of corresponding duration or were left unrestricted as a control group. Arrow symbols (➞) indicate the first sample set at the beginning of the study (i.e., during caging), following samples are marked with (1), (2), or (3) to indicate the first, second and third brood cycles sampled after caging.
Importantly, young mites from non-sampled brood combs were expected to hatch in 20-day intervals during the study (Harbo and Harris 1999), while mites inside the sampled brood combs were lethally removed for investigation of reproductive success. Hence, the sampling dates for the supposed brood cycle of mites after caging refer to the whole mite population in the hive instead of individual mites.
2.2 Brood investigation
All brood samples were stored at – 20 °C until investigation. Overall, 19,084 brood cells (7–12 days post capping) were investigated with respect to their proportionate infestation with mites (i.e., brood infestation) as well as the occurrence of mite non-reproduction (MNR) and recapping (REC) in single infested cells (n = 2579). Brood infestation rates were automatically calculated during the brood investigations. Investigations followed the protocol of the Research Network on Sustainable Bee Breeding (Büchler et al. 2017), more recently also described in Büchler et al. (2020a, b). Accordingly, reproductive failure in terms of MNR was defined by a mother mite solely infesting a cell with either no offspring (infertile), only female offspring (no male) or progeny which was too young in comparison to the developmental stage of the respective host bee pupae (delayed).
2.3 Statistical analysis
All statistical analyses were conducted in the R environment (version 4.1.0, R Core Team 2021). Generalized linear mixed-effect models (glmer) from the binomial family (logit) were used to estimate the probabilities of recapping and non-reproduction on cell level (Bates et al. 2015). The occurrence of recapped cells and non-reproductive mites (including different types of failed reproduction) was considered a response variable. Treatment (i.e., duration of caging) and brood cycle after caging (i.e., subsequent samplings) were implemented as fixed explanatory variables including interactions. In case of non-reproduction and different types of reproductive failure, recapping did overall not contribute to an improved prediction accuracy and was therefore not treated as another explanatory variable. However, this was not the case in a subset of data gained from the first set of brood combs (during caging, Figure 1). In this subset, recapping was included as an explanatory variable alongside with treatment (caged or control) and the respective interactions to investigate the effects on mite reproduction. Tested colonies were considered separate mite populations and thus included as a random factor. Residuals and over-dispersion were analysed using the DHARMa package (Hartig 2021). Subsequent pairwise comparisons among factor levels were performed using Tukey post hoc tests (emmeans (Lenth 2021)).
Due to the data structure, a beta regression (betareg (Cribari-Neto and Zeileis 2010)) was calculated alongside with the functions lrtest (lmtest (Zeileis and Hothorn 2002)) and joint_tests (emmeans (Lenth 2021)) in case of brood infestation. Fixed and random factors were implemented in this model as described above.
3 Results
3.1 Mite non-reproduction (MNR)
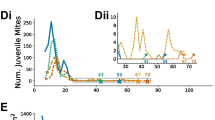
On individual cell level, the predicted probability of MNR was significantly affected by the duration of brood interruption (GLMM: χ2 = 30.4, p < 0.001, df = 3). Overall, the probability of reproductive failure increased with the duration of caging. Longer caging durations also seemed to induce a longer-lasting increase in MNR in the treated colonies compared to control colonies with constant brood rearing (Figure 2).
Predicted probabilities of reproductive failure (MNR) of mother mites in brood combs sampled over the study period (displayed with 95% CI). Symbols below the x-axis refer to the sampling points marked in Figure 1. The duration of 0 days of brood interruption (green symbols) corresponds to the unrestrained control group. The probability of reproductive failure was significantly influenced by the duration of brood interruption (GLMM: χ2 = 30.4, p < 0.001, df = 3) and an interaction of the duration of brood interruption and brood cycle (GLMM: χ2 = 17.52, p = 0.04, df = 9). Different letters indicate significant differences (Tukey HSD, p < 0.05 each) among treatment groups within the respective brood cycle after caging.
There was no clear effect of brood cycle after caging alone. However, we found an interaction effect of brood cycle after queen caging and duration of brood interruption (GLMM: χ2 = 17.52, p = 0.04, df = 9). We therefore report differences between treatment groups separately for each sampling time.
While queens were still caged, the predicted probabilities of MNR in all three groups with brood interruption were significantly higher than in control colonies with undisturbed brood activity, demonstrating a strongly suppressing immediate effect of brood interruption on mite reproduction (p < 0.001 each, Figure 2). Treatment groups with caged queens did not differ in the probability of reproductive failure of mites among each other, while queens were still caged.
In the first brood cycle after caging, probabilities of reproductive failure did not differ between the treatment groups but still tended to be higher in comparison to the control group with continuous brood activity (Figure 2). Here, the highest probabilities of reproductive failure were predicted at this sampling point compared to the other sampling dates within this group (Figure 2).
In the second brood cycle after caging, probabilities of failed reproduction were notably higher in the group with 30 days of brood interruption, which differed significantly from the control group (p < 0.05). Though no other statistically proofed differences between treatment groups were found, probabilities of failed reproduction seemed to be staggered according to the duration of previous brood interruption in the respective groups (Figure 2).
Though the treatment groups with 20 and 30 days of brood interruption still tended to show the highest values in the third brood cycle after caging, predicted probabilities of failed reproduction seemed to be in a comparable range among all groups at this time (Figure 2).
To investigate the immediate effect found during queen caging, colonies with caged queens (i.e., groups 10, 20 and 30) were additionally compared as one treatment group with the uncaged control group at this point in time. Notably, at this date, the caged queens of all treatment groups were restricted in egg laying for the same duration (i.e., 10 days; Figure 1). The predicted probability of MNR in this subset of data was likewise affected by treatment as described above with distinctively higher values in the treatment group (GLMM: χ2 = 15.2, p < 0.001, df = 1). Neither in treated colonies nor in untreated colonies the recapping status of cells showed a significant effect on the occurrence of MNR. However, MNR values tended to be higher in untouched cells respectively (Fig. 1 in supplements).
3.2 Cause of reproductive failure
The underlying causes of MNR (infertile mother, delayed reproduction or missing male) were examined separately. Interestingly, they seemed to be affected differently by the factors investigated (Figure 3). Neither occurrence of missing males nor delayed reproduction seemed to follow a specific pattern related to the duration of brood interruption or the brood cycle sampled (Figure 3b, c).
Predicted probabilities of a infertile mother mites, b delayed reproduction and c missing males in brood combs sampled over the study period (displayed with 95% CI). Pictures showing the respective cause of reproductive failure (a)–(c) in brood cells approximately 9 days post capping: a infertile mother, b delayed reproducing mother and male, c mother and deutonymph daughters without male. Symbols below the x-axis refer to the sampling points marked in Figure 1. The duration of 0 days of brood interruption (green symbols) corresponds to the unrestrained control group. Different letters indicate significant differences (Tukey HSD, p < 0.05 each) among treatment groups within the respective brood cycle after caging. Further test statistics are given in the text.
In contrast to these failures in fertile mites (missing males or delayed reproduction), the predicted probability of infertile mother mites was overall strongly affected by the duration of brood interruption (GLMM: χ2 = 50.29, p < 0.001, df = 3). Similar to the general pattern of MNR, longer durations of brood interruption seemed to have a stronger and longer-lasting effect on the occurrence of infertility (Figure 3a). The probability of infertile mother mites was also affected by the brood cycle sampled (GLMM: χ2 = 16.59, p < 0.001, df = 3) and an interaction of treatment and sampling time (GLMM: χ2 = 28.35, p < 0.001, df = 9). Therefore, pairwise comparisons between treatment groups are reported separately for each sampling time. The probability of infertile mites was remarkably higher in all groups with brood interruption in comparison to the unrestricted control group, while queens were still caged (p < 0.001 each, Figure 3a). In the following brood cycles, the probability of infertile mothers in treatment groups decreased gradually towards that found in the control group. This became particularly apparent when the brood interruption lasted for 30 days (Figure 3a). The probability of infertile mothers in this group decreased in the first and second brood cycles after caging, but was still significantly higher in comparison to the control group (p = 0.004 respectively, Figure 3a). The same trend appeared in the first and second brood cycles after caging for the groups previously restricted in brood rearing for 10 and 20 days (Figure 3a). By the time of the third assumed brood cycle after caging, predicted probabilities for infertility did not differ significantly between groups, but still tended to be higher in the group formerly caged for 30 days (Figure 3a).
As described above for MNR, the underlying causes of reproductive failure were also investigated between the treated and untreated colonies by the time of queen caging. Likewise to the general occurrence of infertile mothers, the probability of infertility in mites at the beginning of the study was strongly affected by treatment (GLMM: χ2 = 20.76, p < 0.001, df = 1) with distinctively higher values in colonies with caged queens (Fig. 2 in supplements). Although the recapping status of cells (GLMM: χ2 = 4.32, p = 0.04, df = 1) as well as the interaction of recapping and treatment (GLMM: χ2 = 4.08, p = 0.04, df = 1) proofed to have a significant effect on the occurrence of infertile mites, this effect seemed to be limited to the uncaged control group. In control colonies, the probability of infertile mites was significantly increased in recapped cells (p < 0.04), while recapping showed no effect on the overall high values of the treatment group (Fig. 2 in supplements). In contrast, the occurrence of delayed reproduction or the absence of males was neither affected by the recapping status of cells, nor the treatment of the respective colony. The probability for delayed reproduction tended to be higher in colonies with caged queens and in untouched cells, while there was no obvious trend in the absence of males (Figs. 3 and 4 in supplements).
Predicted probabilities of recapping (REC) in brood combs sampled over the study period (displayed with 95% CI). Symbols below the x-axis refer to the sampling points marked in Figure 1. The duration of 0 days of brood interruption (green symbols) corresponds to the unrestrained control group. The probability of recapping was significantly influenced by the duration of brood interruption (GLMM: χ2 = 9.41, p = 0.02, df = 3), the brood cycle sampled (GLMM: χ2 = 9.41, p < 0.001, df = 3), as well as an interaction of these factors (GLMM: χ2 = 63.5, p < 0.001, df = 9). Different letters indicate significant differences (Tukey HSD, p < 0.05 each) among treatment groups within the respective brood cycle after caging
3.3 Recapping (REC)
The predicted probability for recapping on individual cell level was significantly affected by treatment (GLMM: χ2 = 9.41, p = 0.02, df = 3). This also applied for the brood cycle sampled (GLMM: χ2 = 9.41, p < 0.001, df = 3), as well as an interaction of these factors (GLMM: χ2 = 63.5, p < 0.001, df = 9). However, differences between groups were only visible while queens were caged in the treatment groups (Figure 4). At this time, all treatment groups with caged queens displayed higher predicted probabilities of recapping than the control group with unrestricted brood rearing. Although this trend was only statistically proven in one of the three caging groups when analysed separately (group 10: p = 0.03, Figure 4), the same effect was generally found when comparing all treated colonies against the control group as described above (GLMM: χ2 = 8.09, p = 0.005, df = 1, Fig. 5 in supplements). In the following brood cycles after caging, predicted probabilities of recapping varied largely within groups and were lacking a clear pattern over time.
Brood infestation of investigated colonies over the study period. Boxplots display median values (inner horizontal lines), 1st and 3rd quartiles (box), minimum and maximum values (whiskers) and outliers (filled dots). Symbols below the x-axis refer to the sampling points marked in Figure 1. The duration of 0 days of brood interruption (green symbols) corresponds to the unrestrained control group. The brood infestation was significantly affected by the duration of brood interruption (GLMM: F = 27.42; p < 0.001), the brood cycle sampled (GLMM: F = 3.02; p = 0.03), as well as an interaction of these factors (GLMM: F = 3.2; p < 0.001). Different letters above boxplots indicate significant differences (Tukey HSD, p < 0.05 each) among treatment groups within the respective brood cycle after caging.
3.4 Brood infestation
The percentage of infested brood cells was significantly affected by treatment (GLMM: F = 27.42, p < 0.001) with overall lower infestation levels in colonies which experienced a brood interruption (Figure 5). The infestation level was also affected by the time of sampling (GLMM: F = 3.02, p = 0.03) and an interaction between both of these factors (GLMM: F = 3.2, p < 0.001). Over the course of the study, this effect was displayed by a constantly increasing brood infestation in the control group (Figure 5). In contrast, groups which experienced a brood interruption before generally showed little variation in the following brood cycles (Figure 5). Notably, brood infestation levels did not differ between groups at the beginning of the study (i.e., first sampling, Figures. 1 and 5). This already changed by the time of the first brood cycle after caging. At this time, control colonies with previously unrestricted brood rearing showed much higher brood infestation levels compared to all treatment groups (p < 0.05 each), which did not differ from each other (Figure 5). The infestation level in control colonies further increased across the second and third brood cycles after caging, while there was no visible increase in the brood infestation of colonies with previous brood interruption (Figure 5). Thus, at the end of the study (i.e., third brood cycle after caging, Figure 1), the brood infestation in all three treatment groups was significantly lower compared to that in the control group with unrestricted brood rearing (p < 0.001 each, Figure 5).
4 Discussion
4.1 Brood interruption reduces reproductive success of mites
Our experiments challenged the hypothesis that brood interruption can alter the reproduction of Varroa destructor.
Honey bee brood is crucial for mite reproduction (Martin 1995a). Thus, brood interruptions, e.g., as a consequence of swarming, seem to be an obstacle for mite reproduction per se. Our results demonstrate that brood interruptions suppress the reproductive success of mites beyond the mere temporary lack of opportunities for cell invasion. Notably, the share of reproductive failure over all treatment groups was highest while the queens were still caged. The mites sampled at this point were suspected to have entered suitable brood cells shortly before the queens’ egg laying stopped. Thus, the observed decrease of reproductive success during brood interruption cannot be explained by a prolonged dispersal phase. Among the known traits associated with increased MNR on colony level, REC is one of the most frequently found behaviours (Grindrod and Martin 2021; Mondet et al. 2020a, b). However, the exact mode of action is still unknown. Natural REC on cell level does not seem to interact directly with the reproductive success of mites infesting the respective cells (Martin et al. 2020; Oddie et al. 2018; Harris et al. 2012; Martin et al. 1997), which overall corresponds with the present results.
Given the complex mating biology of mites in dependence to the honeybee host, it is likely that the importance of single resistance traits also varies over time, e.g., due to seasonal variations in brood rearing or intensity patterns of other worker bee duties. Tison et al. (2022) just recently showed that the Varroa-sensitive hygiene behaviour (VSH) can be less pronounced as a result of increased foraging during strong nectar flows. In our case, the decreasing demand for larvae feeding might have favoured the distinctively increased REC observed during brood interruption in all treatment groups. Although we found no direct effect of REC on MNR, the increase of MNR could be similarly explained by an increased removal of infested brood cells (VSH), as Martin et al. (2020) described REC as a valuable and closely linked proxy for VSH. Though VSH was not investigated in the present study, the observed trend of higher MNR values in untouched (i.e., not recapped) cells additionally supports this hypothesis. The removal of infested brood cells was repeatedly supposed to be biased towards reproductive mites, leading to an increased proportion of MNR in the remaining cells (summarized in Mondet et al. 2020a, b; Oddie et al. 2018).
However, the selective removal of reproductive mites was not confirmed in other studies on VSH (Sprau et al. 2021; Harris et al. 2010), underlining the variability and complexity of linkages between resistance traits. Thus, the occurrence of MNR is most likely affected by a diverse set of traits and interactions varying over time. Although we can only speculate about the underlying mechanisms leading to the spontaneous increased MNR values in the treatment groups, the interruption of brood activity as the initial cause is clearly proven.
The present results also demonstrate long-term effects on MNR. Brood interruptions and correspondingly prolonged dispersal phases in summertime appear to reduce the success of mites’ in following reproductive attempts. Similar effects have been shown for natural winter brood breaks by Otten (1991, see also Otten and Fuchs 1990), as well as for artificially prolonged dispersal phases in summertime (Stürmer and Rosenkranz 1994). In the present study, the suppressing effect on mite reproduction was still visible when the new brood nest comprised all larval and pupal stages again. However, the differences in reproductive success of mite populations in formerly treated and control colonies decreased over time. By the time of the third brood cycle after caging, all treatment and control colonies showed similar MNR values. This recovery effect on population scale fits well to the described number of reproductive cycles for individual Varroa females on colony level, since mites are assumed to reproduce two to three times in a row (Martin and Kemp 1997; Fries and Rosenkranz 1996). Therefore, the gradual recovery of mite reproduction on colony level might be explained by the substitution of old mites (which experienced the brood interruption) by young mites (which hatched afterwards). Likewise, the mite reproduction recovered more quickly after shorter brood interruptions since the proportion of mites forced into a prolonged dispersal phase was correspondingly lower. In addition, the time of sampling showed no direct effect on MNR but significant interactions with the treatment, pointing towards a change in mite population structure rather than a general temporal variability of MNR.
Hence, brood interruptions and prolonged dispersal phases add to various other causes like brood cues and behaviours of adult bees (Mondet et al. 2020a, b) which can alter the reproductive success of mites. Especially the duration of the dispersal phase appears to be important for following reproductive attempts. In fact, the exact role of this part of the mites’ life cycle is still unknown (Rosenkranz et al. 2010; Xie et al. 2016). Early studies showed that mites are able to reproduce up to seven times in a row without a dispersal phase in between (de Ruijter 1987). However, in the first reproductive attempt, this applies most probably only for the oldest of the freshly hatched daughters which already completed the spermatozoa capacitation (Häußermann et al. 2016). Obviously, the dispersal phase in summertime harbours some benefits for the mites since the divided life cycle evolved as an alternative to direct transition into the next reproductive attempt. For example, transportation by the host bees enables the mites to reach new brood cells in both, the current colony by using nurse bees, as well as non-natal colonies by attaching to drifting or robbing foragers (Frey and Rosenkranz 2014; Nazzi and Le Conte 2015; Peck and Seeley 2019). On the other hand, it may also pose dangers for the mites (Pritchard 2016; Rosenkranz et al. 2010; Xie et al. 2016) and is not obligatory for successful reproduction in every case (de Ruijter 1987; Häußermann et al. 2016). Our results show that a decrease in reproductive success on colony level can add to these previously described negative effects for the mites if the dispersal phase is prolonged.
These effects of brood interruptions and corresponding dispersal phase durations should be taken into account in different contexts. The reproductive success of the mite population on colony level holds great importance for the overall infestation and thus the ultimately survival chances of a colony (Rosenkranz et al. 2010). Notably, the here tested durations of 10, 20 and 30 days of brood interruption are field-realistic time spans in naturally swarming colonies. After settling in a new location, it takes at least 10 days for the swarm to produce brood cells old enough for mite invasion (Rosenkranz et al. 2010; Winston 1987). In turn, the remaining part of the colony usually needs between 22 and 30 days after swarming until the young queen starts egg laying (Koeniger et al. 2014; Seeley and Smith 2015; Winston 1987). In this light, natural brood interruptions are usually rated as beneficial for infested colonies (Loftus et al. 2016). Our results suggest that the duration of such swarm-associated brood breaks may also affect mite population development and thus general health status in both parts of the swarmed colony.
Likewise, induced brood interruptions used in beekeeping (Büchler et al. 2020b) may also hold a potential for biotechnical treatments against V. destructor even without a subsequent drug application.
In addition to these implications for practical beekeeping, immediate and long-term effects should be taken into account whenever gathering mites for bioassays used in bee breeding or science. This is done mainly by caging queens for brood interruption in highly infested “mite-donor-colonies” in order to force mites into a dispersal phase in which they can be detached easily from the bees by powdered sugar-shakes (Dietemann et al. 2013). Hence, the afterwards investigated reproduction of mites could be altered by the previously induced brood break. However, in the present study, this effect was less expressed in colonies with shorter durations of queen caging and decreased over time. Therefore, shorter durations of brood interruption in the “mite-donor-colonies” as well as a recovery-phase for the mite population could compensate for the effects of brood interruption when working with artificially infested colonies.
4.2 Brood interruption affects causes of reproductive failure differently
Overall, failed reproduction of mites is characterized by (1) the lack of male offspring; (2) delayed oviposition, desynchronizing age of mite offspring and developmental stage of the host cell; or (3) infertile mother mites. The present results indicate that brood interruptions alter the proportional occurrence of factors causing reproductive failure of mites. Infertility was the most common cause for reproductive failure of mites in treatment groups (48%). It was followed by delayed reproduction (41%) and missing males (11%). This is in contrast to earlier findings by Mondet et al. (2020b) in colonies undisturbed brood activity. By comparing the putative causes of reproductive failure of mites in 106 colonies from six different countries, delayed reproduction was found to be the most common cause (Mondet et al. 2020b). It was followed by infertile mites and mite families without males, while the composition of the respective causes differed significantly between locations (Mondet et al. 2020b). The occurrence of infertility, delayed reproduction and missing males in our control colonies resembled the previously described values (Mondet et al. 2020b). Thus, the differing distribution in treatment groups of the present study seemed to be rather an effect of the brood interruptions than of the location.
Interestingly, the probability for infertile mites was remarkably higher during caging in treatment groups compared to untreated control groups in the present study. Over the course of the subsequent samplings, it converged with those of the control group. It thus showed a similar pattern as the overall reproductive success of mites. Our results strongly indicate that brood activity forms one of the mechanisms affecting the proportion of infertile mother mites. In addition to this effect of brood activity on colony level, the effect of honeybee brood signals on the fertility and reproductive success of mites has been shown for age-related factors inside individual host cells (Frey et al. 2013; Kirrane et al. 2011; Sprau et al. 2021). Those studies showed that the right host age is crucial for oogenesis and proper timing of egg laying in fertile mites. Interestingly, the occurrence of infertile mites, i.e., the absence of egg laying was shown to be higher if mother mites were artificially transferred into older brood cells, even if they already started oogenesis before (Frey et al. 2013). Such transfer situations (from brood cell to brood cell) or mistimed invasions (from dispersal phase to brood cell) could occur under natural circumstances due to resistance behaviours of the bees. Especially, VSH (Kirrane et al. 2011; Mondet et al. 2020a, b) and REC (Grindrod and Martin 2021; Oddie et al. 2018) could potentially lead to such mismatches between host-age and the reproductive status of mites. In addition, the standard method of brood investigation on frozen brood combs, as used in this study, is not capable of the differentiation between mites which already died before sampling from those which were alive at the time of sampling. Thus, some of the non-reproductive mites may simply lacked proper offspring because they died shortly after cell invasion, e.g., as a consequence of recapping. Both hypotheses would explain the significantly higher probabilities of infertile mites in recapped cells of the control group compared to untouched (i.e., not recapped cells) of these colonies. Interestingly, such differences were not found during brood interruption in the treated colonies. In the treatment group, the probability of infertile mothers was overall high in recapped as well as untouched cells. Again, this might be the outcome of selective VSH towards reproducing mites as a consequence of task allocation as discussed in detail in Sect. 4.1.
Although the exact mechanisms leading to different causes of reproductive failure can only be hypothesized, the duration of brood interruption and the time elapsed after caging clearly affected the occurrence of infertile mothers. In addition to these colony level factors, REC altered the occurrence of infertile mothers on cell level at least in some cases. However, the probability for missing males and delayed reproduction appeared to be mostly unaffected by REC and the brood interruptions investigated in the present study.
This again underlines the complexity of the host-parasite interactions between mites and bees as well as the need for further studies on the underlying mechanisms of reproductive failure in mites.
4.3 Queen caging temporarily increases recapping
The uncapping of sealed brood and subsequent recapping of the cells by worker bees (REC) is a common trait in naturally Varroa-surviving honeybee populations (Grindrod and Martin 2021). It also occurs in Varroa-naïve colonies, albeit to a lower degree (Martin et al. 2020). Our results indicate that REC on colony level, likewise to the reproductive success of mites, is also affected by brood interruptions. Notably, the frequency of REC was highest during the caging of queens in treatment groups, which corresponds to a higher probability of reproductive failure of mites at this time. However, in contrast to our findings on mite reproduction, the effect of brood interruptions on REC was only visible during the restriction of egg laying. Thus, it might be a direct but short-term behavioural reaction of the bees to the changing relation of adult bees to young brood cells as shown for other worker duties before (Tison et al. 2022). Nevertheless, this temporal effect on the behaviour of bees might have contributed to a lower reproductive success of mites, both directly as well as in later reproductive attempts as discussed above.
Although REC overall did not show a statistically significant effect on MNR, this could have been masked by comparatively stronger effects of the treatment and sampling time, as supposed for other parameters co-occurring with REC (Oddie et al. 2021) and discussed in Sect. 4.1.
This also corresponds to higher MNR values, which were found in artificially, but not in naturally recapped cells (Oddie et al. 2018). However, Varroa-surviving honeybee populations frequently display higher levels of MNR than susceptible colonies (Grindrod and Martin 2021; Locke 2016), which was recently shown to be directly affected by the recapping frequency of infested cells on colony level (Oddie et al. 2021). This likewise points to a more complex effect of REC on MNR, which may be sometimes hidden on colony level.
Overall, the mite depressing effects of REC are increasingly gaining attention, promoting this trait as an appropriate criterion for selection towards Varroa-resistance (Büchler et al. 2020a, b; Oddie et al. 2021). The present results show that the expression of this trait is also linked to brood interruptions in the colonies investigated, which should be taken into account when measuring recapping rates. Since there was no clear pattern in the occurrence of REC over the course of subsequent samples after caging, it is likely that the behaviour of individual colonies was altered additionally by other environmental factors (Oddie et al. 2021).
4.4 Long-lasting reduction of brood infestation after brood interruption
Over the course of the study, brood interruptions had a clear effect on infestation levels of worker brood. Infestation levels were found to be high (on average 12%) but comparable among treatment groups and the untreated control colonies in the beginning of the study. Although the infestation level of control colonies tended to be higher during the caging of queens in the treatment groups, the mite loads did not differ significantly between groups at this time. In contrast, mite loads were remarkably higher in the control group compared to the three treatment groups already by the time of the first brood cycle after caging. Consequently, this difference increased continuously until the end of the study. This was partly expected due to the interrupted mite population growth in treatment groups, in contrast to the continuous brood activity in the also highly infested control group. Similar differences in infestation levels are known to be of great importance for the health status of naturally swarming colonies (Loftus et al. 2016; Seeley and Smith 2015). Nevertheless, the higher reproductive success of mites with continuous brood activity found in the present study most probably enhanced this effect additionally. Again, this seemed to be an effect of brood interruption, since recent studies did not find any direct effects of infestation levels on reproductive success of mites on colony level (Mondet et al. 2020b). Thus, reproductive success in single-infested cells seems to be altered by brood interruptions but not by the level of brood infestation itself. On the other hand, it is clear that the reproductive success of mites directly influences the mite population growth and is thus an important factor for the infestation level of brood cells (Nazzi and Le Conte 2015; Rosenkranz et al. 2010). Overall, the sharp contrast in infestation levels between colonies with brood interruption and those without points towards a general benefit of well-timed brood breaks for colony health.
5 Conclusion
The interruption of brood rearing clearly alters the reproductive success of mites, the recapping frequency and the brood infestation on colony level. It is therefore not only important for the survival of honeybee colonies, but may also interfere with measurements of resistance parameters.
Our results show for the first time that inhibiting the honeybee queen from egg laying for durations which are comparable to naturally occurring brood breaks can significantly reduce the probability for mite reproduction. In this case, brood interruptions mainly affected the proportion of infertile mother mites. This applies not only for the time of caging, but also for following brood cycles. After the brood interruption, however, the mite population seems to recover over time and regains normal reproductive abilities. How long this recovery takes seems to depend on the duration of the former brood interruption.
Reproductive failure of mites is one of the most accounted traits in honeybee science and breeding for resistance against Varroa destructor. The present study underlines the complexity of this trait as well as the challenges in comparable measurements of mite reproduction.
Despite the importance for standardized data acquisition, the lower reproductive success as well as the decreased mite infestation on colony level once again point to the beneficial aspects of summer brood interruptions in practical beekeeping.
Data availability
The datasets generated and analysed during the present study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67(1). https://doi.org/10.18637/jss.v067.i01
Beetsma J, Boot WJ, Calis J (1999) Invasion behaviour of Varroa jacobsoni Oud.: from bees into brood cells. Apidologie 30(2–3):125–140. https://doi.org/10.1051/apido:19990204
Boot WJ, Calis JNM, Beetsma J (1992) Differential periods of Varroa mite invasion into worker and drone cells of honey bees. Exp Appl Acarol 16(4):295–301
Boot WJ, Calis JNM, Beetsma J (1993) Invasion of Varroa jacobsons into honey bee brood cells: a matter of chance or choice? J of Api Res 32(3–4):167–174. https://doi.org/10.1080/00218839.1993.11101302
Boot WJ, Calis JNM, Beetsma J (1995a) Does time spent on adult bees affect reproductive success of Varroa mites? Entomol Exp Appl 75(1):1–7. https://doi.org/10.1111/j.1570-7458.1995.tb01903.x
Boot WJ, Schoenmaker J, Calis JNM, Beetsma J (1995b) Invasion of Varroa jacobsoni into drone brood cells of the honey bee. Apis Mellifera Apidologie 26(2):109–118
Büchler R, Berg S, Le Conte Y (2010) Breeding for resistance to Varroa destructor in Europe. Apidologie 41(3):393–408. https://doi.org/10.1051/apido/2010011
Büchler R, Costa C, Mondet F, Kezic N, Kovacic M (2017) Screening for low Varroa mite reproduction (SMR) and recapping in European honey bees [online] https://www.beebreeding.net/wp-content/uploads/2017/11/RNSBB_SMR-recapping_protocol_2017_09_11.pdf (Accessed on 24 March 22)
Büchler R, Kovačić M, Buchegger M, Puškadija Z, Hoppe A, Brascamp EW (2020a) Evaluation of traits for the selection of Apis mellifera for resistance against Varroa destructor. Insects 11(9). https://doi.org/10.3390/insects11090618
Büchler R, Uzunov A, Kovačić M, Prešern J, Pietropaoli M et al (2020b) Summer brood interruption as integrated management strategy for effective Varroa control in Europe. J of Api Res 59(5):764–773. https://doi.org/10.1080/00218839.2020.1793278
Cervo R, Bruschini C, Cappa F, Meconcelli S, Pieraccini G, Pradella D, Turillazzi S (2014) High Varroa mite abundance influences chemical profiles of worker bees and mite-host preferences. J Exp Biol 217(Pt 17):2998–3001. https://doi.org/10.1242/jeb.099978
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Soft 34(2). https://doi.org/10.18637/jss.v034.i02
de Ruijter A (1987) Reproduction of Varroa jacobsoni during successive brood cycles of the honeybee. Apidologie 18(4):321–326
Dietemann V, Pflugfelder J, Anderson D, Charrière J-D, Chejanovsky N et al (2012) Varroa destructor : research avenues towards sustainable control. J of Api Res 51(1):125–132. https://doi.org/10.3896/IBRA.1.51.1.15
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B et al (2013) Standard methods for varroa research. J of Api Res 52(1):1–54. https://doi.org/10.3896/IBRA.1.52.1.09
Donzé G, Guerin PM (1994) Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav Ecol Sociobiol 34(5):305–319. https://doi.org/10.1007/BF00197001
Donzé G, Herrmann M, Bachofen B, Guerin PM (1996) Effect of mating frequency and brood cell infestation rate on the reproductive success of the honeybee parasite Varroa jacobsoni. Ecol Entomol 21(1):17–26. https://doi.org/10.1111/j.1365-2311.1996.tb00261.x
Eynard SE, Sann C, Basso B, Guirao AL, Le Conte Y, Servin B, Tison L, Vignal A, Mondet F (2020) Descriptive analysis of the Varroa non-reproduction trait in honey bee colonies and association with other traits related to Varroa resistance. Insects 11(8). https://doi.org/10.3390/insects11080492
Frey E, Odemer R, Blum T, Rosenkranz P (2013) Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). J Invertebr Pathol 113(1):56–62. https://doi.org/10.1016/j.jip.2013.01.007
Frey E, Rosenkranz P (2014) Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J Econ Entomol 107(2):508–515. https://doi.org/10.1603/ec13381
Fries I, Hansen H, Imdorf A, Rosenkranz P (2003) Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie 34(4):389–397. https://doi.org/10.1051/apido:2003032
Fries I, Rosenkranz P (1996) Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp Appl Acarol 20(2):103–112. https://doi.org/10.1007/BF00051156
Fuchs S (1990) Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie 21(3):193–199. https://doi.org/10.1051/apido:19900304
Garrido C, Rosenkranz P (2003) The reproductive program of female Varroa destructor mites is triggered by its host. Apis Mellifera Exp Appl Acarol 31(3–4):269–273. https://doi.org/10.1023/b:appa.0000010386.10686.9f
Grindrod I, Martin SJ (2021) Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Proceedings Biological Sciences 288(1956):20211375. https://doi.org/10.1098/rspb.2021.1375
Harbo JR, Harris JW (1999) Heritability in honey bees (Hymenoptera: Apidae) of characteristics associated with resistance to Varroa jacobsoni (Mesostigmata: Varroidae). ec 92(2):261–265. https://doi.org/10.1093/jee/92.2.261
Harris JW (2007) Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged ≤ five days post capping. J of Api Res 46(3):134–139. https://doi.org/10.1080/00218839.2007.11101383
Harris JW, Danka RG, Villa JD (2010) Honey bees (Hymenoptera: Apidae) with the trait of Varroa sensitive hygiene remove brood with all reproductive stages of Varroa mites (Mesostigmata: Varroidae). Ann Entomol Soc Am 103(2):146–152
Harris JW, Danka RG, Villa JD (2012) Changes in infestation, cell cap condition, and reproductive status of Varroa destructor (Mesostigmata: Varroidae) in brood exposed to honey bees with Varroa sensitive hygiene. Annls Entomol Soc Am 105:512–518
Hartig F (2021) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.3. https://CRAN.R-project.org/package=DHARMa
Häußermann CK, Ziegelmann B, Rosenkranz P (2016) Spermatozoa capacitation in female Varroa destructor and its influence on the timing and success of female reproduction. Exp Appl Acarol 69(4):371–387. https://doi.org/10.1007/s10493-016-0051-4
Kirrane MJ, de Guzman LI, Rinderer TE, Frake AM, Wagnitz J, Whelan PM (2011) Asynchronous development of honey bee host and Varroa destructor (Mesostigmata: Varroidae) influences reproductive potential of mites. J Econ Entomol 104(4):1146–1152. https://doi.org/10.1603/ec11035
Koeniger G, Koeniger N, Tiesler F-K (2014) Paarungsbiologie und Paarungskontrolle bei der Honigbiene. Buschhausen Druck und Verlagshaus, Herten, Westf
Le Conte Y, Meixner MD, Brandt A, Carreck NL, Costa C, Mondet F, Büchler R (2020) Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 11(12). https://doi.org/10.3390/insects11120873
Lenth RV (2021) emmeans: estimated marginal means, aka least-squares means. R package version 1.7.1–1. https://CRAN.R-project.org/package=emmeans
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47(3):467–482. https://doi.org/10.1007/s13592-015-0412-8
Loftus JC, Smith ML, Seeley TD (2016) How honey bee colonies survive in the wild: testing the importance of small nests and frequent swarming. PloS one 11(3):e0150362. https://doi.org/10.1371/journal.pone.0150362
Martin SJ (1995a) Ontogenesis of the mite Varroa jacobsoni Oud. in drone brood of the honeybee Apis mellifera L. under natural conditions. Exp Appl Acarol 19(4):199–210. https://doi.org/10.1007/BF00130823
Martin SJ (1995b) Reproduction of Varroa jacobsoni in cells of Apis mellifera containing one or more mother mites and the distribution of these cells. J of Api Res 34(4):187–196. https://doi.org/10.1080/00218839.1995.11100904
Martin SJ, Hawkins GP, Brettell LE, Reece N, Correia-Oliveira ME, Allsopp MH (2020) Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 51(3):369–381. https://doi.org/10.1007/s13592-019-00721-9
Martin SJ, Kemp D (1997) Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera) colonies. J Apic Res 36(3-4):113–123. https://doi.org/10.1080/00218839.1997.11100937
Mondet F, Kim SH, de Miranda JR, Beslay D, Le Conte Y, Mercer AR (2016) Specific cues associated with honey bee social defence against Varroa destructor infested brood. Sci Rep 6:25444. https://doi.org/10.1038/srep25444
Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y (2020a) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int J Parasitol 50(6–7):433–447. https://doi.org/10.1016/j.ijpara.2020.03.005
Mondet F, Parejo M, Meixner MD, Costa C, Kryger P et al (2020b) Evaluation of suppressed mite reproduction (SMR) reveals potential for Varroa resistance in European honey bees (Apis mellifera L.). Insects 11(9). https://doi.org/10.3390/insects11090595
Nazzi F, Milani N (1996) The presence of inhibitors of the reproduction of Varroa jacobsoni Oud. (Gamasida: Varroidae) in infested cells. Exp Appl Acarol 20(11):617–623. https://doi.org/10.1007/BF00053325
Nazzi F, Le Conte Y (2015) Ecology of Varroa destructor, the major ectoparasite of the Western honey bee, Apis mellifera Annu Rev Entomol. https://doi.org/10.1146/annurev-ento-010715-023731
Oddie M, Büchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, de Miranda JR, Mondet F, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8(1):7704. https://doi.org/10.1038/s41598-018-26001-7
Oddie M, Burke A, Dahle B, Le Conte Y, Mondet F, Locke B (2021) Reproductive success of the parasitic mite (Varroa destructor) is lower in honeybee colonies that target infested cells with recapping. Sci Rep 11(1):9133. https://doi.org/10.1038/s41598-021-88592-y
Otten C (1991) Vergleichende Untersuchungen zum Populationswachstum von Varroa jocobsoni OUD. in Völkern von Apis mellifera L. unterschiedlicher geographischer Herkunft
Otten C, Fuchs S (1990) Seasonal variations in the reproductive behavior of Varroa jacobsoni in colonies of A. mellifera carnica, A. m. ligustica and A. m. mellifera - Western Germany Bee Research Institutes Seminar. Report on the Meeting at Adelsdorf, 28–30 March 1990. Apidologie 21(4):323–377. https://doi.org/10.1051/apido:19900407
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PloS one 14(6):e0218392. https://doi.org/10.1371/journal.pone.0218392
Piou V, Tabart J, Urrutia V, Hemptinne JL, Vétillard A (2016) Impact of the Phoretic phase on reproduction and damage caused by Varroa destructor (Anderson and Trueman) to its host, the European honey bee (Apis mellifera L.). PloS one 11(4):e0153482. https://doi.org/10.1371/journal.pone.0153482
Pritchard DJ (2016) Grooming by honey bees as a component of varroa resistant behavior. J of Api Res 55(1):38–48. https://doi.org/10.1080/00218839.2016.1196016
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rosenkranz P, Garrido C (2004) Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Evolutionary, Mechanistic and Environmental Approaches to Chemically-Mediated Interactions 14(3–4). https://doi.org/10.1007/s00049-004-0278-0
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):119. https://doi.org/10.1016/j.jip.2009.07.016
Sammataro D, Gerson U, Needham G (2000) Parasitic mites of honey bees: life history, implications, and impact. Annu Rev Entomol 45:519–548. https://doi.org/10.1146/annurev.ento.45.1.519
Seeley TD, Smith ML (2015) Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor - Springer. Apidologie 46:716–727. https://doi.org/10.1007/s13592-015-0361-2
Sprau L, Hasselmann M, Rosenkranz P (2021) Reproduction of Varroa destructor does not elicit varroa sensitive hygiene (VSH) or recapping behaviour in honey bee colonies (Apis mellifera). Apidologie 52(6):1048–1059. https://doi.org/10.1007/s13592-021-00886-2
Stürmer M, Rosenkranz P (1994) 22. Die Bedeutung der phoretischen Phase für die Oogenese von Varroa jacobsoni. Apidologie 25(5):453–455
Tison L, Riva C, Maisonnasse A, Kretzschmar A, Hervé MR, Le Conte Y, Mondet F (2022) Seasonal and environmental variations influencing the Varroa Sensitive Hygiene trait in the honey bee. Entomologia Generalis 42(1):1–10
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36(7):592–606. https://doi.org/10.1016/j.pt.2020.04.004
Vanengelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, Frazier M, Frazier J, Cox-Foster D, Chen Y, Underwood R, Tarpy DR, Pettis JS (2009) Colony collapse disorder: a descriptive study. PloS one 4(8):e6481. https://doi.org/10.1371/journal.pone.0006481
Winston ML (1987) The biology of the honey bee. Harvard University Press
Xie X, Huang ZY, Zeng Z (2016) Why do Varroa mites prefer nurse bees? Sci Rep 6:28228. https://doi.org/10.1038/srep28228
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2(3):7–10. https://CRAN.R-project.org/doc/Rnews/
Acknowledgements
We thank Hilda Strasser, Sadam Alasad and Daniel Brechensbauer for assistance in data collection and colony maintenance. For statistical advice and comments on the early version of the manuscript, we thank Douglas Sponsler.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support programme.
Author information
Authors and Affiliations
Contributions
All authors contributed to the elaboration of the study design. Sampling, statistical analysis and preparation of the first draft were performed by MG. In the following, RS, RB and MG commented on earlier versions of the manuscript and contributed in the writing process. The final manuscript version has been read and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Peter Rosenkranz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gabel, M., Scheiner, R. & Büchler, R. Immediate and long-term effects of induced brood interruptions on the reproductive success of Varroa destructor. Apidologie 54, 20 (2023). https://doi.org/10.1007/s13592-023-00998-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-00998-x