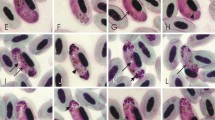
Abstract
Haemosporidians (Haemosporida) are cosmopolitan in birds. Over 250 species of these blood parasites have been described and named; however, molecular markers remain unidentified for the great majority of them. This is unfortunate because linkage between DNA sequences and identifications based on morphological species can provide important information about patterns of transmission, virulence, and evolutionary biology of these organisms. There is an urgent need to remedy this because few experts possess the knowledge to identify haemosporidian species and few laboratories are involved in training these taxonomic skills. Here, we describe new mitochondrial cytochrome b markers for the polymerase chain reaction (PCR)-based detection of four widespread species of avian Haemoproteus (Haemoproteus hirundinis, Haemoproteus parabelopolskyi, Haemoproteus pastoris, Haemoproteus syrnii) and 1 species of Plasmodium (Plasmodium circumflexum). Illustrations of blood stages of the reported species are given, and morphological and phylogenetic analyses identify the DNA lineages that are associated with these parasites. This study indicates that morphological characters, which have been traditionally used in taxonomy of avian haemosporidian parasites, have a phylogenetic value. Perspectives on haemosporidian diagnostics using microscopic and PCR-based methods are discussed, particularly the difficulties in detection of light parasitemia, coinfections, and abortive parasite development. We emphasize that sensitive PCR amplifies more infections than can be transmitted; it should be used carefully in epidemiology studies, particularly in wildlife parasitology research. Because molecular studies are describing remarkably more parasite diversity than previously expected, the need for traditional taxonomy and traditional biological knowledge is becoming all the more crucial. The linkage of molecular and morphological approaches is worth more of the attention of researchers because this approach provides new knowledge for better understanding insufficiently investigated lethal diseases caused by haemosporidian infections, particularly on the exoerythrocytic (tissue) and vector stages. That requires close collaboration between researchers from different fields with a common interest.



Similar content being viewed by others
References
Beadell JS, Ishtiaq F, Covas R, Melo M, Warren BH, Atkinson CT, Bensch S, Graves GR, Jhala YV, Peirce MA, Rahmani AR, Fonseca DM, Fleischer RC (2006) Global phylogeographic limits of Hawaii’s avian malaria. Proc Biol Sci 273:2935–2944. doi:10.1098/rspb.2006.3671
Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins SL, Graves GR, Fleischer RC (2009) Host associations and evolutionary relationships of avian blood parasites from West Africa. Int J Parasitol 39:257–266. doi:10.1016/j.ijpara.2008.06.005
Bennett GF, Whiteway M, Woodworth-Lynas C (1982) A host-parasite catalogue of the avian haematozoa. Occasional Papers in Biology. Memorial University of Newfoundland
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenicity. J Nat Hist 27:993–1001. doi:10.1080/00222939300770621
Bensch S, Stjenman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 276:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Waldenström J, Jonzén N, Westerdahl H, Hansson B, Sejberg D, Hasselquist D (2007) Temporal dynamics and diversity of avian malaria parasites in a single host species. J Anim Ecol 76:112–122. doi:10.1111/j.1365-2656.2006.01176.x
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Bensch S, Hellgren O, Križanauskienė A, Palinauskas V, Valkiūnas G, Outlaw D, Ricklefs RE (2013) How can we determine the molecular clock of malaria parasites? Trends Parasitol 29:363–369. doi:10.1016/j.pt.2013.03.011
Bonneaud C, Sepil I, Milá B, Buermann W, Pollinger J, Sehgal RNM, Valkiūnas G, Iezhova TA, Saatchi S, Smith TB (2009) The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. J Trop Ecol 25:439–447. doi:10.1017/S0266467409006178
Braga ÉM, Silveira P, Belo NO, Valkiūnas G (2011) Recent advances in the study of avian malaria: an overview with an emphasis on the distribution of Plasmodium spp. in Brazil. Mem Inst Oswaldo Cruz 06(Suppl 1):3–11
Cannell BL, Krasnec KV, Campbell K, Jones HI, Miller RD, Stephens N (2013) The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor). Vet Parasitol 197:74–84. doi:10.1016/j.vetpar.2013.04.025
Carlson JS, Martínez-Gómez JE, Valkiūnas G, Loiseau C, Bell DA, Sehgal RN (2013) Diversity and phylogenetic relationships of hemosporidian parasites in birds of Socorro Island, México, and their role in the re-introduction of the Socorro Dove (Zenaida graysoni). J Parasitol 99:270–276. doi:10.1645/GE-3206.1
Dimitrov D, Zehtindjiev P, Bensch S (2010) Genetic diversity of avian blood parasites in SE Europe: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitol 55:201–209. doi:10.2478/s11686-010-0029-z
Dimitrov D, Valkiūnas G, Zehtindjiev P, Ilieva M, Bensch S (2013) Molecular characterization of haemosporidian parasites (Haemosporida) in yellow wagtail (Motacilla flava), with description of in vitro ookinetes of Haemoproteus motacillae. Zootaxa 3666:369–381. doi:10.11646/zootaxa.3666.3.7
Dimitrov D, Zehtindjiev P, Bensch S, Ilieva M, Iezhova T, Valkiūnas G (2014) Two new species Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst Parasitol 87:135–151. doi:10.1007/s11230-013-9464-1
Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalis IH (2008) Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J Vet Diagn Investig 20:304–313. doi:10.1177/104063870802000307
Ferrell ST, Snowden K, Marlar AB, Garner M, Lung NP (2007) Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildl Med 38:309–316. doi:10.1638/1042-7260(2007)038[0309:FHIIMA]2.0.CO;2
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell Scientific Publications, Oxford
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp 41:95–98
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Hellgren O, Križanauskienė A, Valkiūnas G, Bensch S (2007) Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). J Parasitol 93:889–896. doi:10.1645/GE-1051R1.1
Hellgren O, Kutzer M, Bensch B, Valkiūnas G, Palinauskas V (2013) Identification and characterization of the merozoite surface protein 1 (msp1) gene in a host-generalist avian malaria parasite, Plasmodium relictum (lineages SGS1 and GRW4) with the use of blood transcriptome. Malar J 12:381. doi:10.1186/1475-2875-12-381
Ilgūnas M, Palinauskas V, Iezhova TA, Valkiūnas G (2013) Molecular and morphological characterization of two avian malaria parasites (Haemosporida: Plasmodiidae), with description of Plasmodium homonucleophilum n. sp. Zootaxa 3666:49–61. doi:10.11646/zootaxa.3666.1.5
Imura T, Suzuki Y, Ejiri H, Sato Y, Ishida K, Sumiyama D, Murata K, Yukawa M (2012) Prevalence of avian haematozoa in wild birds in a high-altitude forest in Japan. Vet Parasitol 183:244–248. doi:10.1016/j.vetpar.2011.07.027
Ishak HD, Dumbacher JP, Anderson NL, Keane JJ, Valkiūnas G, Haig SM, Tell LA, Sehgal RNM (2008) Blood parasites in owls with conservation implications for the Spotted Owl (Strix occidentalis). PloS one 3(5):e2304. doi:10.1371/journal.pone.0002304
Karadjian G, Puech MP, Duval L, Chavatte JM, Snounou G, Landau I (2013) Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite 20:32. doi:10.1051/parasite/2013031
Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA, Valkiūnas G (2013) Plasmodium relictum (lineages pSGS1 and pGRW11): complete synchronous sporogony in mosquitoes Culex pipiens pipiens. Exp Parasitol 133:454–461. doi:10.1016/j.exppara.2013.01.008
Kim KS, Tsuda Y, Sasaki T, Kobayashi M, Hirota Y (2009) Mosquito blood–meal analysis for avian malaria study in wild bird communities: laboratory verification and application to Culex sasai (Diptera: Culicidae) collected in Tokyo, Japan. Parasitol Res 105:1351–1357. doi:10.1007/s00436-009-1568-9
Križanauskienė A, Iezhova TA, Palinauskas V, Chernetsov N, Valkiūnas G (2012) Haemoproteus nucleocondensus n. sp. (Haemosporida, Haemoproteidae) from a Eurasian songbird, the Great Reed Warbler Acrocephalus arundinaceus. Zootaxa 3441: 36–46. doi: http://dx.doi.org/10.11646/zootaxa.3616.1.7
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163. doi:10.1093/bib/5.2.150
Levin II, Valkiūnas G, Iezhova TA, O’Brien SL, Parker PG (2012) Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J Parasitol 98:847–854. doi:10.1645/GE-3007.1
Levin II, Zwiers P, Deem SL, Geest EA, Higashiguchi JM, Iezhova TA, Jiménez-Uzcátegui G, Kim DH, Morton JP, Perlut NG, Renfrew RB, Sari EHR, Valkiunas G, Parker PG (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Conserv Biol 27:1366–1377. doi:10.1111/cobi.12127
Loiseau C, Iezhova TA, Valkiūnas G, Chasar A, Hutchinson A, Buermann W, Smith TB, Sehgal RN (2010) Spatial variation of haemosporidian parasite infection in African rainforest bird species. J Parasitol 96:21–29. doi:10.1645/GE-2123.1
Mantilla JS, González AD, Valkiūnas G, Moncada LI, Matta NE (2013) Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol Res 112:4193–4204. doi:10.1007/s00436-013-3611-0
Martínez J, Martínez-De La Puente J, Herrero J, Del Cerro S, Lobato E, Rivero-De Aguilar J, Vásquez RA, Merino S (2009) A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology 136:713–722. doi:10.1017/S0031182009006118
Martinsen ES, Paperna I, Schall JJ (2006) Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology 133:279–288. doi:10.1017/S0031182006000424
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. doi:10.1016/j.ympev.2007.11.012
Marzal A, de Lopes F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545. doi:10.1007/s00442-004-1757-2
Marzal A, Bensch S, Reviriego M, Balbontin J, De Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21:979–987. doi:10.1111/j.1420-9101.2008.01545.x
Matta NE, Lotta IA, Valkiūnas G, González AD, Pacheco MA, Escalante AA, Moncada LI, Rodríguez-Fandiño OA (2014) Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol Res 113:457–468. doi:10.1007/s00436-013-3675-x
Merino S, Hennicke J, Martínez J, Ludynia K, Torres R, Work TM, Stroud S, Masello JF, Quillfeldt P (2012) Infection by Haemoproteus parasites in four species of frigatebirds and the description of a new species of Haemoproteus (Haemosporida, Haemoproteidae). J Parasitol 98:388–397. doi:10.1645/GE-2415.1
Njabo K, Cornel AJ, Sehgal RN, Loiseau C, Buermann W, Harrigan RJ, Pollinger J, Valkiūnas G, Smith TB (2009) Coquillettidia (Culicidae, Diptera) mosquitoes are natural vectors of avian malaria in Africa. Malaria J 8:193. doi:10.1186/1475-2875-8-193
Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950–952. doi:10.3201/eid1705.101618
Outlaw DC, Ricklefs RE (2009) On the phylogenetic relationships of haemosporidian parasites from raptorial birds (Falconiformes and Strigiformes). J Parasitol 95:1171–1176. doi:10.1645/GE-1982.1
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae). Zootaxa 1626:39–50
Palinauskas V, Iezhova TA, Križanauskienė A, Markovets MY, Bensch S, Valkiūnas G (2013a) Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): a pathogenic avian parasite. Parasitol Int 62:358–363. doi:10.1016/j.parint.2013.03.006
Palinauskas V, Križanauskienė A, Iezhova TA, Bolshakov CV, Jönsson J, Bensch S, Valkiūnas G (2013b) A new method for isolation of purified genomic DNA from haemosporidian parasites inhabiting nucleated red blood cells. Exp Parasitol 133:275–280. doi:10.1016/j.exppara.2012.12.003
Palmer JL, McCutchan TF, Vargas FH, Deem SL, Cruz M, Hartman DA, Parker PG (2013) Seroprevalence of malarial antibodies in Galapagos penguins (Spheniscus mendiculus). J Parasitol 99:770–776. doi:10.1645/12-57.1
Pérez-Tris J, Hellgren O, Križanauskienė A, Waldenström J, Secondi J, Bonneaud C, Fjeldså J, Hasselquist D, Bensch S (2007) Within-host speciation of malaria parasites. PloS one 2(2):e235. doi:10.1371/journal.pone.0000235
Perkins SL (2014) Malaria’s many mates: past, present and future of the systematics of the order Haemosporida. J Parasitol 100:11–25. doi:10.1645/13-362.1
Perkins SL, Austin CC (2009) Four new species of Plasmodium from New Guinea lizards: integrating morphology and molecules. J Parasitol 95:424–433. doi:10.1645/GE-1750.1
Piersma T, van der Velde M (2012) Dutch House Martins Delichon urbicum gain blood parasite infections over their lifetime, but do not seem to suffer. J Ornithol 153:907–912. doi:10.1007/s10336-012-0826-2
Ricklefs RE, Fallon SM, Bermingham E (2004) Evolutionary relationships, cospeciation and host switching in avian malaria parasites. Syst Biol 53:111–119. doi:10.1080/10635150490264987
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Santiago-Alarcon D, Bloch R, Rolshausen G, Schaefer HM, Segelbacher G (2011) Prevalence, diversity, and interaction patterns of avian haemosporidians in a four-year study of blackcaps in a migratory divide. Parasitology 138:824–835. doi:10.1017/S0031182011000515
Schaer J, Perkins SL, Decher J, Leendertz FH, Fahr J, Weber N, Matuschewski K (2013) High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc Natl Acad Sci U S A 110:17415–17419. doi:10.1073/pnas.1311016110
Sehgal RNM, Valkiūnas G, Iezhova TA, Smith TB (2006) Blood parasites of chickens in Uganda and Cameroon with molecular descriptions of Leucocytozoon schoutedeni and Trypanosoma gallinarum. J Parasitol 92:1336–1343. doi:10.1645/GE-927R.1
Shurulinkov P, Ilieva M (2009) Spatial and temporal differences in the blood parasite fauna of passerine birds during the spring migration in Bulgaria. Parasitol Res 104:1453–1458. doi:10.1007/s00436-009-1349-5
Svoboda A, Marthinsen G, Turčoková L, Lifjeld JT, Johnsen A (2009) Identification of blood parasites in Old World warbler species from the Danube river delta. Avian Dis 53:634–636. doi:10.1637/8842-040409-Case.1
Swofford DL (2002) PAUP*: Phylogenetic analysis using parsimony (*and other methods). Version 4.0b. Sinauer Associates, Sunderland, Massachusetts
Synek P, Albrecht T, Vinkler M, Schnitzer J, Votýpka J, Munclinger P (2013) Haemosporidian parasites of a European passerine wintering in South Asia: diversity, mixed infections and effect on host condition. Parasitol Res 112:1667–1677. doi:10.1007/s00436-013-3323-5
Tanigawa M, Sato Y, Ejiri H, Imura T, Chiba R, Yamamoto H, Kawaguchi M, Tsuda Y, Murata K, Yukawa M (2013) Molecular identification of avian haemosporidia in wild birds and mosquitoes on Tsushima Island, Japan. J Vet Med Sci 75:319–326. doi:10.1292/jvms.12-0359
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G, Iezhova TA, Shapoval AP (2003) High prevalence of blood parasites in hawfinch Coccothraustes coccothraustes. J Nat Hist 37:2647–2652. doi:10.1080/002229302100001033221
Valkiūnas G, Bensch S, Iezhova TA, Križanauskienė A, Hellgren O, Bolshakov CV (2006) Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422
Valkiūnas G, Križanauskienė A, Iezhova TA, Hellgren O, Bensch S (2007) Molecular phylogenetic analysis of circumnuclear hemoproteids (Haemosporida: Haemoproteidae) of sylviid birds, with a description of Haemoproteus parabelopolskyi sp. nov. J Parasitol 93:680–687. doi:10.1645/GE-1102R.1
Valkiūnas G, Atkinson CT, Bensch S, Sehgal RNM, Ricklefs RE (2008a) Parasite misidentifications in GenBank: how to minimize their number? Trends Parasitol 24:247–248. doi:10.1016/j.pt.2008.03.004
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S (2008b) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. doi:10.1645/GE-1570.1
Valkiūnas G, Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA (2013a) Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res 112(6):2159–2169. doi:10.1007/s00436-013-3375-6
Valkiūnas G, Palinauskas V, Križanauskienė A, Bernotienė R, Kazlauskienė R, Iezhova TA (2013b) Further observations on in vitro hybridization of hemosporidian parasites: patterns of ookinete development in Haemoproteus spp. J Parasitol 99:124–136. doi:10.1645/GE-3226.1
Valkiūnas G, Kazlauskienė R, Bernotienė R, Bukauskaitė D, Palinauskas V, Iezhova TA (2014a) Haemoproteus infections (Haemosporida, Haemoproteidae) kill bird-biting mosquitoes. Parasitol Res 113:1011–1018. doi:10.1007/s00436-013-3733-4
Valkiūnas G, Palinauskas V, Ilgūnas M, Bernotienė R, Iezhova TA (2014b) In vitro development of Haemoproteus parasites: the efficiency of reproductive cells increase during simultaneous sexual process of different lineages. Parasitol Res. doi:10.1007/s00436-014-3782-3
Ventim R, Morais J, Pardal S, Mendes L, Ramos JA, Pérez-Tris J (2012) Host-parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology 139:310–316. doi:10.1017/S0031182011002083
Waldenström J, Bensch S, Kiboi S, Hasselquist D, Ottosson U (2002) Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol Ecol 11:1545–1554
Walker D, Garnham PC (1972) Aberrant Leucocytozoon infection in parakeets. Vet Rec 91:70–72. doi:10.1136/vr.91.3.70
Yohannes E, Križanauskienė A, Valcu M, Bensch S, Kempenaers B (2009) Prevalence of malaria and related haemosporidian parasites in two shorebird species with different winter habitat distribution. J Ornithol 150:287–291. doi:10.1007/s10336-008-0349-z
Zehtindjiev P, Križanauskienė A, Scebba S, Dimitrov D, Valkiūnas G, Hegemann A, Tieleman BI, Bensch S (2011) Haemosporidian infections in skylarks (Alauda arvensis): a comparative PCR-based and microscopy study on the parasite diversity and prevalence in southern Italy and the Netherlands. Eur J Wildl Res 58:335–344. doi:10.1007/s10344-011-0586-y
Zehtindjiev P, Ivanova K, Mariaux J, Georgiev BB (2013) First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitol Res 112:3509–3515. doi:10.1007/s00436-013-3533-x
Acknowledgments
We would like to thank the staff of the biological station “Rybachy” of the Zoology Institute of Russian Academy of Sciences and Kalimok biological station of the Institute of Biodiversity and Ecosystem Research at the Bulgarian Academy of Sciences for assistance in the field. We are grateful to Aneliya Bobeva, Vilius Armalis, and Martin P. Marinov for their help during field work. This study complies with the current laws of Lithuania, Bulgaria, and Russia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valkiūnas, G., Palinauskas, V., Ilgūnas, M. et al. Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 113, 2251–2263 (2014). https://doi.org/10.1007/s00436-014-3880-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3880-2