Abstract
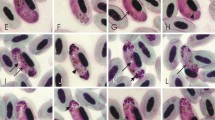
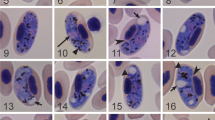
Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) are described: Haemoproteus (Parahaemoproteus) homovelans n. sp. from Grey-faced Woodpecker, Picus canus Gmelin, and Haemoproteus (Parahaemoproteus) concavocentralis n. sp. recorded in Hawfinch, Coccothraustes coccothraustes (Linnaeus), both sampled in Bulgaria. The morphology of the gametocytes and their host-cells are described and mitochondrial cytochrome b (cyt b) gene sequences are generated. Haemoproteus homovelans possesses circumnuclear gametocytes lacking volutin granules. This parasite is particularly similar to Haemoproteus velans Coatney & Roudabush, 1937 also possessing circumnuclear gametocytes that are, however, overfilled with volutin. Haemoproteus concavocentralis can be readily distinguished from all described avian haemoproteids due to the presence of an unfilled concave space between the central part of advanced gametocytes and erythrocyte nucleus. Bayesian phylogenetic analyses of 40 haemosporidian cyt b lineages showed close relationships of H. concavocentralis (hHAWF2) with a group of Haemoproteus spp. possessing gametocytes that are pale-stained with Giemsa. The lineage hPICAN02 of H. homovelans clustered with parasites infecting non-passerine birds. Phylogenetic analyses support the current subgeneric classification of the avian haemoproteids and suggest that cyt b lineage hPIPUB01 (GenBank EU254552) has been incorrectly assigned to Haemoproteus picae Coatney & Roudabush, 1937, a common parasite of corvid birds (Passeriformes). This study emphasises the importance of combining molecular techniques and light microscopy in the identification and field studies of avian haemosporidian parasites. Future development of barcodes for molecular identification of haemoproteids will allow better diagnostics of these infections, particularly in veterinary studies addressing insufficiently investigated tissue pathology caused by these parasites.



Similar content being viewed by others
References
Atkinson, C. T. (2008). Haemoproteus. In: Atkinson, C. T., Thomas, N. J., & Hunter B. C. (Eds) Parasitic Diseases of Wild Birds. Ames, Iowa, USA: Wiley-Blackwell, pp. 13–35.
Atkinson, C. T., Forrester, D. J., & Greiner, E. C. (1988). Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. Journal of Parasitology, 74, 228–239.
Beadell, J. S., Gering, E., Austin, J., Dumbacher, J. P., Peirce, M. A., Pratt, T. K., Atkinson, C., & Fleischer, R. C. (2004). Prevalence and differential host specificity of two avian blood parasite genera in the Australo-Papuan region. Molecular Ecology, 13, 3829–3844.
Bennett, G. F., Peirce, M. A., & Ashford, R. W. (1993). Avian haematozoa: Mortality and pathogenicity. Journal of Natural History, 27, 993–1001.
Bennett, G. F., & Campbell, A. G. (1972). Avian Haemoproteidae. III. Description of Haemoproteus fallisi n. sp. and a review of the haemoproteids of the family Turdidae. Canadian Journal of Zoology, 50, 1269–1275.
Bensch, S., Stjernman, M., Hasselquist, D., Ostman, O., Hansson, B., Westerdahl, H., & Pinheiro, R. T. (2000). Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society B, 267, 1583–1589.
Bensch, S., Pérez-Tris, J., Waldenström, J., & Hellgren, O. (2004). Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution, 58, 1617–1621.
Bensch, S., Hellgren, O., & Pérez-Tris, J. (2009). MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources, 9, 1353–1358.
Bobeva, A. B., Zehtindjiev, P., Bensch, S., & Radrova, J. (2013). A survey of biting midges of the genus Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) in NE Bulgaria, with respect to transmission of avian haemosporidians. Acta Parasitolgica 58, 585–591.
BWPi 2.0. (2006). The Birds of the Western Palaearctic Interactive, 2006 Upgrade. DVD Birdguides, Shrewsbury.
Cannell, B. L., Krasnec, K. V., Campbell, K., Jones, H. I., Miller, R. D., & Stephens, N. (2013). The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor). Veterinary Parasitology, 197, 74–84.
Dimitrov, D., Zehtindjiev, P., & Bensch, S. (2010). Genetic diversity of avian blood parasites in SE Europe: Cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitologica, 55, 201–209.
Ferrell, S. T., Snowden, K., Marlar, A. B., Garner, M., & Lung, N. P. (2007). Fatal hemoprotozoal infections in multiple avian species in a zoological park. Journal of Zoo and Wildlife Medicine, 38, 309–316.
Garnham, P. C. C. (1966). Malaria parasites and other Haemosporidia. Oxford: Blackwell Scientific Publications.
Garvin, M. C., Homer, B. L., & Greiner, E. C. (2003). Pathogenicity of Haemoproteus danilewskyi, Kruse, 1890, in blue jays (Cyanocitta cristata). Journal of Wildlife Diseases, 39, 161–169.
Gilman, S., Blumstein, D. T., & Foufopoulos, J. (2007). The effect of hemosporidian infections on white-crowned sparrow singing behaviour. Ethology, 113, 437–445.
Godfrey, R. D., Fedynich, A. M., & Pence, D. B. (1987). Quantification of hematozoa in blood smears. Journal of Wildlife Diseases, 23, 558–565.
Greiner, E. C., Mandal, A. K., & Nandi, N. C. (1977). Haemoproteus bennetti sp. n. and a review of the haemoproteids from the Picidae (Woodpeckers). Journal of Parasitology, 63, 651–656.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor v. 5.0.9. Nucleic Acids Symposium, 41, 95–98.
Hellgren, O., Waldenström, J., & Bensch, S. (2004). A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. Journal of Parasitology, 90, 797–802.
Hellgren, O., Križanauskienė, A., Valkiūnas, G., & Bensch, S. (2007). Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). Journal of Parasitology, 93, 889–896.
Iezhova, T. A., Valkiūnas, G., Loiseau, C., Smith, T. B., & Sehgal, R. N. M. (2010). Haemoproteus cyanomitrae sp. nov. (Haemosporida, Haemoproteidae) from a widespread African songbird, the olive sunbird Cyanomitra olivacea. Journal of Parasitology, 96, 137–143.
Iezhova, T. A., Dodge, M., Sehgal, R. N. M., Smith, T. B., & Valkiūnas, G. (2011). New avian Haemoproteus species (Haemosporida: Haemoproteidae) from African birds, with a critique of the use of host taxonomic information in Hemoproteid classification. Journal of Parasitology, 97, 682–694.
Križanauskienė, A., Hellgren, O., Kosarev, V., Sokolov, L., Bensch, S., & Valkiūnas, G. (2006). Variation in host specificity between species of avian haemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. Journal of Parasitology, 92, 1319–1324.
Križanauskienė, A., Pérez-Tris, J., Palinauskas, V., Bensch, S., & Valkiūnas, G. (2010). Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology, 137, 217–227.
Križanauskienė, A., Iezhova, T. A., Chernetsov, N., Palinauskas, V., & Valkiūnas, G. (2012). Haemoproteus nucleocondensus n. sp. (Haemosporida, Haemoproteidae) from a Eurasian songbird, the Great Reed Warbler Acrocephalus arundinaceus. Zootaxa, 3441, 36–46.
Križanauskienė, A., Iezhova, T. A., Sehgal, R. N. M., Carlson, J. S., Palinauskas, V., Bensch, S., & Valkiūnas, G. (2013). Molecular characterization of Haemoproteus sacharovi (Haemosporida, Haemoproteidae), a common parasite of columbiform birds, with remarks on classification of haemoproteids of doves and pigeons. Zootaxa, 3613, 085–094.
Levin, I. I., Valkiūnas, G., Santiago-Alarcon, D., Cruz, L. L., Iezhova, T. A., O’Brien, S. L., Hailer, F., Dearborn, D., Scheiber, E. A., Fleischer, R. C., Ricklefs, R. E., & Parker, P. G. (2011). Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: Evidence from molecular and morphological studies, with a description of Haemoproteus iwa. International Journal for Parasitology, 41, 1019–1027.
Levin, I. I., Valkiūnas, G., Iezhova, T. A., O’Brien, S. L., & Parker, P. G. (2012). Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Laridae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. Journal of Parasitology, 98, 847–854.
Levin, I. I., Zwiers, P., Deem, S. L., Geest, E. A., Higashiguchi, J. M., Iezhova, T. A., Jiménez-Uzcátegui, G., Kim, D. H., Morton, J. P., Perlut, N. G., Renfrew, R. B., Sari, E. H. R., Valkiūnas, G., & Parker, P. G. (2013). Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Conservation Biology, 27, 1366–1377.
Levine, N. D., & Campbell, G. R. (1971). A check-list of the species of the genus Haemoproteus (Apicomplexa, Plasmodiidae). Journal of Parasitology, 18, 475–484.
Martínez, J., Martínez-De La Puente, J., Herrero, J., Del Cerro, S., Lobato, E., Rivero-De Aguilar, J., Vásquez, R. A., & Merino, S. (2009). A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology, 136, 713–722.
Martinsen, E. S., Paperna, I., & Schall, J. J. (2006). Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology, 133, 279–288.
Martinsen, E. S., Perkins, S., & Schall, J. J. (2008). A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Molecular Phylogenetics and Evolution, 47, 261–273.
Marzal, A., De Lopes, F., Navarro, C., & Møller, A. P. (2005). Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia, 142, 541–545.
Marzal, A., Reviriego, M., Hermosell, I. G., Balbontín, J., Bensch, S., Relinque, C., Rodríguez, L., Garcia-Longoria, L., & de Lope, F. (2013). Malaria infection and feather growth rate predict reproductive success in house martins. Oecologia, 171, 853–861.
Merino, S., Hennicke, J., Martínez, J., Ludynia, K., Torres, R., Work, T. M., Stroud, S., Masello, J. F., & Quillfeldt, P. (2012). Infection by Haemoproteus parasites in four species of frigatebirds and the description of a new species of Haemoproteus (Haemosporida, Haemoproteidae). Journal of Parasitology, 98, 388–397.
Møller, A. P., & Nielsen, J. T. (2007). Malaria and risk of predation: a comparative study of birds. Ecology, 88, 871–881.
Nylander, J. A. A. (2004). MrModeltest v2. Evolutionary Biology Centre, Uppsala, Program distributed by the author. Available from: http://www.abc.se/~nylander/. Accessed 18 February 2012.
Olias, P., Wegelin, M., Zenker, W., Freter, S., Gruber, A. D., & Klopfleisch, R. (2011). Avian Malaria Deaths in Parrots, Europe. Emergent Infection Diseases, 17, 950–952.
Palinauskas, V., Kosarev, V., Shapoval, A., Bensch, S., & Valkiūnas, G. (2007). Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae). Zootaxa, 1626, 39–50.
Palinauskas, V., Iezhova, T. A., Križanauskienė, A., Markovets, M. Y., Bensch, S., & Valkiūnas, G. (2013). Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): a pathogenic avian parasite. Parasitology International, 62, 358–363.
Peirce, M. A. (1981). Distribution and host–parasite check-list of the haematozoa of birds in Western Europe. Journal of Natural History, 15, 419–458.
Perkins, S. L., & Schall, J. J. (2002). A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. Journal of Parasitology, 88, 972–978.
Ricklefs, R. E., & Fallon, S. M. (2002). Diversification and host switching in avian malaria parasites. Proceeding of the Royal Society B, 269, 885–892.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Sambrook, J., Fritch, F. J., & Maniatis, T. (2002). Molecular cloning: a laboratory manual. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press.
Santiago-Alarcon, D., Outlaw, D. C., Ricklefs, R. E., & Parker, P. G. (2010). Phylogenetic relationships of haemosporidian parasites in New World Columbiformes, with emphasis on the endemic Galapagos dove. International Journal for Parasitology, 40, 463–470.
Shurulinkov, P., & Golemansky, V. (2002). Haemoproteids (Haemosporida: Haemoproteidae) of wild birds in Bulgaria. Acta Protozoologica, 41, 359–374.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution, 28, 2731–2739.
Valkiūnas, G. (2005). Avian malaria parasites and other haemosporidia. Boca Raton, Florida: CRC Press.
Valkiūnas, G., Liutkevičius, G., & Iezhova, T. A. (2002). Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera: Ceratopogonidae). Journal of Parasitology, 88, 864–868.
Valkiūnas, G., Iezhova, T. A., & Shapoval, A. P. (2003). High prevalence of blood parasites in Hawfinch Coccothraustes coccothraustes. Journal of Natural History, 37, 2647–2652.
Valkiūnas, G., Bensch, S., Iezhova, T. A., Križanauskienė, A., Hellgren, O., & Bolshakov, C. V. (2006). Nested cytochrome b PCR diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. Journal of Parasitology, 92, 418–422.
Valkiūnas, G., Križanauskienė, A., Iezhova, T. A., Hellgren, O., & Bensch, S. (2007). Molecular phylogenetic analysis of circumnuclear hemoproteids (Haemosporida: Haemoproteidae) of Sylviid birds, with a description of Haemoproteus parabelopolskyi sp. nov. Journal of Parasitology, 93, 680–687.
Valkiūnas, G., Atkinson, C. T., Bensch, S., Sehgal, R. N. M., & Ricklefs, R. E. (2008a). Parasite misidentifications in GenBank: how to minimise their number? Trends in Parasitology, 24, 247–248.
Valkiūnas, G., Iezhova, T. A., Križanauskienė, A., Palinauskas, V., Sehgal, R. N. M., & Bensch, S. (2008b). A comparative analysis of microscopy and PCR-based detection methods for blood parasites. Journal of Parasitology, 94, 1395–1401.
Valkiūnas, G., Iezhova, T. A., Loiseau, C., Chasar, A., Smith, T. B., & Sehgal, R. N. M. (2008c). New species of haemosporidian parasites (Haemosporida) from African rainforest birds, with remarks on their classification. Parasitology Research, 103, 1213–1228.
Valkiūnas, G., Iezhova, T. A., Loiseau, C., & Sehgal, R. N. M. (2009). Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. Journal of Parasitology, 95, 1512–1515.
Waldenström, J., Bensch, S., Hasselquist, D., & Östman, Ö. (2004). A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. Journal of Parasitology, 90, 191–194.
Acknowledgements
The authors are grateful to Dr. Robert Adlard and Dr. Mal Bryant, Queensland Museum and Science Centre, Australia for providing the type-material of H. velans. We thank Dr. Vaidas Palinauskas for assistance in the laboratory. We are grateful to both anonymous reviewers for the valuable comments and suggestions. Part of the laboratory work was possible because of the facilities created in the Institute of Biodiversity and Ecosystem Research during the project CEBDER funded by the Bulgarian National Science Fund. This study was supported by the European Union Structural Funds project “Postdoctoral Fellowship Implementation in Lithuania” (VP-3.1-ŠMM-01-V-02-004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimitrov, D., Zehtindjiev, P., Bensch, S. et al. Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst Parasitol 87, 135–151 (2014). https://doi.org/10.1007/s11230-013-9464-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-013-9464-1