Abstract
Purpose
This article summarizes natural products that target the MAPK-signaling pathway in cancer therapy. The classification, chemical structures, and anti-cancer mechanisms of these natural products are elucidated, and comprehensive information is provided on their potential use in cancer therapy.
Methods
Using the PubMed database, we searched for keywords, including “tumor”, “cancer”, “natural product”, “phytochemistry”, “plant chemical components”, and “MAPK-signaling pathway”. We also screened for compounds with well-defined structures that targeting the MAPK-signaling pathway and have anti-cancer effects. We used Kingdraw software and Adobe Photoshop software to draw the chemical compound structural diagrams.
Results
A total of 131 papers were searched, from which 85 compounds with well-defined structures were selected. These compounds have clear mechanisms for targeting cancer treatment and are mainly related to the MAPK-signaling pathway. Examples include eupatilin, carvacrol, oridonin, sophoridine, diosgenin, and juglone. These chemical components are classified as flavonoids, phenols, terpenoids, alkaloids, steroidal saponins, and quinones.
Conclusions
Certain MAPK pathway inhibitors have been used for clinical treatment. However, the clinical feedback has not been promising because of genomic instability, drug resistance, and side effects. Natural products have few side effects, good medicinal efficacy, a wide range of sources, individual heterogeneity of biological activity, and are capable of treating disease from multiple targets. These characteristics make natural products promising drugs for cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a serious health challenge globally, with 2020 statistics showing that more than 19 million new cases and approximately 10 million deaths occurred. It is estimated that new cancer cases worldwide will increase by approximately 50% in 20 years (Mao et al. 2022). Currently, breast cancer is the most common cancer, with approximately 2.3 million new patients each year. The next most frequent types are lung, colorectal, prostate, and stomach cancers. However, lung cancer is the leading cause of cancer-related mortality, with approximately 1.8 million deaths each year, followed by colorectal, liver, stomach, and breast cancers (Sung et al. 2021). Evidence from clinical research suggests that a poor diet, obesity, and insufficient exercise habits may increase cancer risk. Many cancer cases can be treated, with current common treatment methods, including chemotherapy, hormone therapy, immunotherapy, and targeted therapy (Siegel et al. 2022; Rock et al. 2022). Therefore, identifying new therapeutic agents with specific effects on different tumor types is necessary.
MAPK pathway is a highly conserved tertiary kinase model (He and Meng 2020) that is mainly composed of MAPKKK, MAPKK, and MAPK (Park and Baek 2022). Intracellular and extracellular signals stimulate the upstream kinase MAPKKK, which responds by activating the intermediate kinase MAPKK. This then activates the downstream kinase MAPK (Lee et al. 2020). In mammals, there are more than a dozen proteins in the model of the tertiary kinase of MAPK. The four most common subprotein families include the ERK1/2, JNK, p38, and ERK5 families (Cargnello and Roux 2011). MAPK pathway signaling can impact many biological processes in eukaryotic cells and can regulate different cellular activities, including proliferation, differentiation, and migration, by transducing extracellular signals (Guo et al. 2022; Kent et al. 2020; Brägelmann et al. 2021).
However, to our knowledge, no preclinical or clinical research on natural products targeting MAPK-signaling pathways in cancer has been published. Therefore, this article summarizes the components of natural products and the role of the MAPK pathway in various cancers, as well as elucidating the mechanisms of their potential use for cancer treatment.
Major MAPK-signaling pathways
The three main MAPK-related-signaling pathways in cells are the classical MAPK pathway, the JNK and p38 MAPK pathway, and the ERK5 pathway.
Overactivation of the classical MAPK pathway (Ras/RAF/MEK/ERK (MAPK) pathway) leads to more than 40% of cancer cases (Yuan et al. 2020). RAS is a gene family that is commonly mutated in cancer. The most frequent mutation is KRAS, such as pancreatic ductal adenocarcinomas and colorectal carcinomas (Drosten and Barbacid 2020). RAS is usually activated on the membrane downstream of the growth factor receptors. RAS contains three gene isoforms: H-RAS, K-RAS, and N-RAS. Although they have highly homologous sequences, they have distinct functions that lead to different physiological functions (Moore et al. 2020; Yuan et al. 2020). RAF protein family kinases include three isoforms: RafA, RafB, RafC, as well as two close pseudokinases (KSR1/2). In addition, BRAF mutations occur in approximately 8% of cancers, which are very common in melanomas (Drosten and Barbacid 2020). In this classical pathway, the RAS mutation rate is the highest (22%), followed by BRAF (8%) and MEK (< 1%), while ERK mutations are sporadic (Yaeger and Corcoran 2019). The three-layered MAPK-signaling cascade is initiated when RTK and RAS are activated. The three RAF subtypes and downstream MEK1/2 and ERK1/2 from a constitute-signaling module to guide a series of physiological functions (Ullah et al. 2022).
The p38 MAPK pathway plays essential roles in signaling cascade responses. In mammals, it has four p38 kinase members: p38α, p38β, p38γ, and p38δ. The expression patterns of the upstream activator and downstream effector differ (Cheng et al. 2020). p38 MAPK is involved in regulating cell proliferation, growth, and apoptosis. It is usually activated by MKK3 and MKK6 kinases, but can also be phosphorylated through MKK4 kinase, which is an activator of JNK. When the p38 protein is activated, it is usually transferred from the cytoplasm to the nucleus to regulate downstream-signaling molecules (Sui et al. 2014). JNK has three subtypes: JNK1, JNK2, and JNK3. JNK1 and JNK2 are widely distributed in tissues, but JNK3 is mainly limited to neuronal tissues, testis, and cardiac myocytes (Cargnello and Roux 2011). They respond to various stressors, such as DNA-damaging agents and oxidative stress (Xie et al. 2018). Activation of JNK MAPK is similar to the p38 MAPK process, mainly occurring via upstream kinases MKK4 and MKK7. This requires dual phosphorylation of Thr and Tyr residues within a conserved Thr–Pro–Tyr motif in their activation loops (Cargnello and Roux 2011). This supports the regulation of various cellular processes, including autophagy, differentiation, and proliferation.
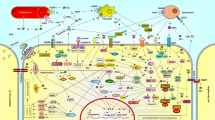
ERK5 is another protein kinase of the triple MAPK-signaling cascade. It has three types: ERKa, ERKb, and ERKc. ERK5 has a C-terminal extension that includes a nuclear localization signal and a transcriptional transactivation domain (Cristea et al. 2020). Its activation is achieved through MEK5 phosphorylation. When its kinase domain is activated, ERK5 can phosphorylate multiple residues between its C-terminus. S753 and T732 have been characterized, and phosphorylation-specific antibodies have been produced against them (Cook and Lochhead 2022). ERK5 has diverse expression levels in various tissues, with high expression levels in the brain, thymus, and spleen. This signaling pathway participates in cell growth, differentiation, and decay and is related to cancer (Tubita et al. 2022). The three main transduction pathways of the MAPK-signaling pathway are shown in Fig. 1.
Three major transduction pathways of the MAPK-signaling pathway. When chemical molecules of natural products enter the cell membrane, they interact with key target proteins of the MAPK-signaling pathway. These in turn activate the cascade response of the MAPK-signaling pathway, thereby regulating apoptosis in cancer cells
MAPK in homeostasis
When regulating energy homeostasis, glycogen is usually the first choice for cells to store and use energy. Some research has shown that lipocytes scale up energy consumption by consistently expressing UCP1 in response to long-term sympathetic activation. ROS are produced during glycogen turnover. ROS can activate p38 MAPK, which drives the expression of UCP1. However, in the presence of an ROS scavenger, p38 activation and UCP1 expression levels were significantly decreased. While it remains unknown if ROS can directly activate p38, this work indirectly demonstrated that p38 is an essential link for regulating energy homeostasis (Keinan et al. 2021). Furthermore, the p38 MAPK pathway is also closely related to the maintenance of lipid homeostasis. In vitro experiments showed that hypothalamic ependymal–glia (tanycytes) can produce and secrete Fgf21 to regulate lipid homeostasis. Tanycytes can oxidize palmitate, produce ROS, and trigger the p38 MAPK pathway, which is critical for tanycytic Fgf21 expression upon palmitate exposure. When the p38 MAPK inhibitor SB203580 was used, the expression and secretion of palmitate-induced Fgf21 were prevented in cultured tanycytes. Thus, the p38 MAPK pathway is necessary for Fgf21 secretion and expression, highlighting its importance in lipid homeostasis (Geller et al. 2019). The MEK–ERK pathway is also related to cell homeostasis, with Feng et al. (2021) demonstrating that inhibition of excess ROS or the MEK/ERK pathway could save the survival of PDK1-deficient Treg cells.
The MAPK-signaling pathway is also involved in maintaining protein homeostasis. In the ECM, TMEM2 decomposes glycosaminoglycan HA and then changes ER stress resistance and stress sensitivity. The latter depends on the cell surface receptor CD44 and ERK and p38 pathways, which is mainly achieved through the ERK and p38 orthologues MPK-1 and PMK-1. In addition, human studies and experiments of C. elegans prove that deletion of ERK or p38 inhibits the advantage of TMEM2 and that HTMEM2 relies on PMK-1 to regulate immune homeostasis (Schinzel et al. 2019).
According to one study, AICD is a form of apoptosis. When the source of inflammation has been eliminated, the organism can use this process to shut down the T-cell response and then maintain lymphocyte homeostasis. This begins with the activation of JNK1 and ERK1/2 induced by AICD, which then leads to activation of the apoptogenic factor BIM and fission protein DRP1. In this process, ERK1/2 upregulates the Bim-L and Bim-S subtypes and cooperates with JNK1 to activate DRP1. JNK1 does not affect the activation of ERK1/2 during AICD. However, when mitochondrial structural alterations begin, ERK1/2 needs to maintain JNK1 activation via phosphorylation to maintain lymphatic T-cell homeostasis (Simula et al. 2020).
Tadokoro et al. (2018) discuss how diet affects HSC homeostasis, an animal model fed with a high-fat diet showed that, Spred1 can negatively regulate the RAS–MAPK-signaling pathway to protect HSC homeostasis. In addition, Spred1 can regulate HSC homeostasis through the ERK-signaling pathway, because the serum SCF levels will be increased from obesity. This excessive SCF can overactivate the ERK pathway, resulting in abnormal self-renewal of HSCs and maintenance of hemopoietic homeostasis. Furthermore, Drosophila ISCs play an essential role in gut homeostasis. If ISCs grow abnormally, gut homeostasis can be destroyed and cause a series of diseases, such as colon cancer. However, the Egfr–Ras85D/Ras1 MAPK-signaling pathway is the main element in ISC proliferation. When SH3PX1-dependent autophagy function is lost, Egfr will be surrounded by Rab11-endosomes at the cell surface. This hyperactivates the MAPK/ERK-signaling pathway and volitionally provokes ISC hyperplasia. In human cell experiments, it was also shown that inhibition of autophagy could increase ERK phosphorylation levels. Therefore, it is important to determine how the MAPK pathway maintains gut homeostasis for the treatment of gut diseases (Zhang et al. 2019a, b).The major protein kinases of the MAPK-signaling pathway and their protein–ligand pockets are shown in Fig. 2.
MAPK-signaling pathway and cancer
MAPK signal transduction is an important pathway that adjusts cellular life processes and abnormal changes. Various MAPK pathway-related protein kinases have been found in cancer research, and their genetic modification is closely connected with cancer. Activation of this pathway can trigger microscopic tumor cell proliferation, differentiation, and apoptosis. For example, approximately 25% of mutations in human cancer are related to KRAS, but KRAS-targeted therapeutic agents are lacking. MEK and RAF1 in the MAPK-signaling pathway may be potentially critical targets for blocking signaling from mutated KRAS (Drosten and Barbacid 2020; Wang et al. 2019). Liu et al. (2022) clarified the connection between MAPK signaling and ovarian cancer cells, demonstrating that the activation of this pathway induced resistance to palbociclib. Stramucci et al. (2019) demonstrated that MKK3 can activate the p38 pathway to maintain survival signal transduction in colorectal cancer cells. The MAPK-signaling pathway is often triggered in various cancers and is considered a promising direction for cancer therapeutic development (Herman et al. 2022; Li et al. 2022; Ravichandran et al. 2022; Zhang et al. 2022).
Currently, there are seven drugs targeting the MAPK-signaling pathway for cancer treatment worldwide, including selumetinib sulfate, pirfenidone, cobimetinib, trametinib, binimetinib, donafenib tosylate, and tivozanib (Behr et al. 2021; Ascierto et al. 2023; Gershenson et al. 2022). Other cancer therapeutics related to MAPK signal transduction are in the early clinical trial development. Examples include the ERK1/2 inhibitor LY3214996, selumetinib, ulixertinib, fluorouracil, PDR001, bortezomib, and a combination of trametinib and dabrafenib (Gross et al. 2020; Jung et al. 2022; Bouffet et al. 2023). Other therapeutic agents in various clinical trial phases are shown in Table 1.
MAPK-signaling pathway in breast cancer
Breast cancer is a frequently observed tumor in women. While approximately 70–80% of early non-metastatic patients can be cured, advanced cases with organ metastasis are currently considered incurable. Metastatic factors are a major cause of patient death (Harbeck et al. 2019; Xu et al. 2020). Breast cancer is a genetically heterogeneous disease, with six basic molecular subtypes. Nulliparity, having fewer children, and later age at menopause are all related to an increased risk of breast cancer (Pashayan et al. 2020; Pedrosa et al. 2018).
Wen et al. (2019) suggested that the MAPK-signaling pathway may be a key target for treating breast cancer. Cancer-associated fibroblast-derived IL-32 specifically combines with integrin β3 via the RGD motif, then activates the p38 pathway. This upregulates the expression levels of epithelial–mesenchymal transition markers and promotes cancer cell invasion (Wen et al. 2019; Elwakeel et al. 2022). Another study explored the mechanism of the IncRNA prncr1 and MAPK-signaling pathway in breast cancer. The study emphasized that this lncRNA competes with miR-377, which causes an increase in CCND2 expression levels. The MEK protein kinase can then be activated and support tumor cell growth (Ouyang et al. 2021).
Sequencing technologies have helped clarify many genomic changes that occur in breast cancer, such as mutations in FOXA1, PIK3CA, ERBB2, and SPOP E78K. These may serve as transfer or treatment functions. Among these mutations, ERBB2 is a hotspot mutation in breast cancer that can induce RAS/RAF/MAPK-signaling pathway activity, and the NF1 deletion mutation is also involved in MAPK activation (Razavi et al. 2018). Clinical data have shown that some MAPK-signaling pathway inhibitors are being studied. For example, mirametinib is used alone or combined with fulvestrant. However, the research results of certain drugs are not satisfactory. For instance, the results of the combination of fulvestrant and selumetinib suggested that this approach did not improve patient prognosis. In addition, selumetinib may reduce the effectiveness of endocrine therapy, and there is also a problem of poor tolerance with monotherapy doses (Zaman et al. 2015).
MAPK-signaling pathway in colorectal cancer
The incidence and mortality rate of colorectal cancer are increasing every year. The standard treatments for this disease are chemotherapy, radiotherapy, and surgery (Johdi and Sukor 2020). However, the recurrence rate of colorectal cancer is high. After radical resection of the primary tumor, approximately 30–40% of colorectal cancer patients will develop metastases. Therefore, a therapeutic method to prevent recurrence is needed (Cañellas-Socias et al. 2022).
Changes in the MAPK-signaling pathway are common in colorectal cancer. For example, through animal experiments, Bai et al. (2022) showed that smoke can induce gut microbiota dysbiosis and damage the intestinal shielding function, which may activate the carcinogenic ERK/MAPK pathway in the colon epithelium. In addition, ubiquitin-specific protease can accelerate the proliferation and growth of colorectal cancer cells via the ERK/MAPK pathway by stabilizing protein phosphatase 1 catalytic subunit alpha (Sun et al. 2019).
Most colorectal cancer cells depend on EGFR/KRAS/BRAF/MAPK-signaling pathway activation (Johnson et al. 2022). However, in KRAS and BRAF-mutant colorectal tumors, direct targeting of the MAPK-signaling pathway to inhibit ERK activation has been clinically unsuccessful, but the use of a combination approach that included EGFR inhibition has achieved promising results (Ponsioen et al. 2021). Recently, EGFR inhibitors, such as cetuximab, leucovorin, and oxaliplatin, have been used to treat colorectal cancer (Parseghian et al. 2023; Elez et al. 2022). In colorectal cancer, RAF is the most commonly mutated gene in this pathway. This occurs in approximately 10% of patients with metastatic colorectal cancer (Grothey et al. 2021). However, according to clinical research, only approximately half of cancer patients respond to single-drug treatment with BRAF inhibitors. This is because the adaptive feedback of the MAPK pathway mediated by EGFR is reactivated. Therefore, targeting this adaptive feedback pathway in such colorectal cancer cases can enhance the curative effect. However, MAPK reactivation remains a key issue with primary and acquired drug resistance (Corcoran et al. 2018).
MAPK-signaling pathway in pancreatic cancer
Hereditary factors are a main contributor to pancreatic cancer development. The incidence of pancreatic cancer is high in North America and Europe, and smoking, alcohol consumption, and a high cholesterol diet may all contribute to the risk of developing pancreatic cancer. Pancreatic cancer patients are usually categorized into resectable, marginally resectable, locally advanced, and metastatic groups according to the degree of disease. Surgical resection is currently the main treatment method. Systemic chemotherapy combinations are often administered to patients with advanced disease (Mizrahi et al. 2020).
The microenvironment of pancreatic cancer has received increasing attention and consists mainly of cancer cells, stromal cells and extracellular components (Ren et al. 2018a, b). PDAC is the most commonly observed intraepithelial tumor of the pancreas (Vincent et al. 2011). Bryant et al. (2019) suggested that PDAC characteristics include KRAS and autophagy-dependent tumor growth, demonstrating that the inhibition of KRAS and ERK could increase autophagic flow. From their data, the authors believe that a drug combination that could simultaneously inhibit ERK and upregulate the autophagy process would be a possibly effective therapeutic approach. Ravichandran et al. (2022) treated PDAC with the MEK inhibitor trimetinib. MAPK-signaling pathway inhibition resulted in decreased c-MYC expression levels and an increase in MiT/TFE-dependent lysosomal biogenesis. The destruction of ferritinophagy synergizes and cooperates with the KRAS/MAPK-signaling pathway to inhibit PDAC growth, highlighting a key target of metabolic dependency.
In another study, Lin et al. (2021) also showed that the MAPK pathway is connected to pancreatic cancer cells. PA cells were significantly inhibited when a PKC/MEK inhibitor was added to cancer cells overexpressing TRPM2. The results suggested that TRPM2 possibly directly activates PKCα through calcium or indirectly triggers PKCε and PKCδ through increased DAG. This then activates the MAPK-signaling pathway to promote PA growth.
Pancreatic cancer is frequently at an advanced stage when diagnosed (Klein 2021) and has developed chemotherapy resistance, which contributes to the unsatisfactory treatment of pancreatic cancer patients. Targeting the MAPK-signaling pathway may provide a new option for treating pancreatic cancer.
MAPK-signaling pathway in gastric cancer
Gastric cancer is a highly molecularly and phenotypically heterogeneous disease. Helicobacter pylori infection, pickled food, and smoking are all risk factors for gastric cancer (Smyth et al. 2020). Differences in tumor biology between Eastern and Western countries increase the complexity of international standard treatments. The effective ways to treat gastric cancer include systemic chemotherapy, immunotherapy, surgery, targeted therapy, and radiotherapy. Drugs approved for the treatment of gastric cancer include ramucirumab and pembrolizumab (Joshi and Badgwell 2021).
The MAPK-signaling pathway is connected with multiple factors in gastric cancer development, metastasis, and treatment. The secondary messenger Ca2+ is a crucial regulatory factor during gastric cancer metastasis. Calcium release activates the calcium regulator (ORAI2), which can enhance cancer cell metastasis by inducing FAK-mediated MAPK/ERK pathway activation (Wu et al. 2021). HDACs are a hot topic, with inhibition of HDACs being recognized as a cancer treatment method. Functional measurements showed that Class IIA (HDAC4) is upregulated in gastric cancer cells and related to poor prognosis. HDAC4 inhibits the transcription of ATG4B in an MEF2A-driven manner, prevents MEKK3 from p62-dependent autophagic degradation, and then activates p38 protein kinase. HDAC4 plays a carcinogenic role in gastric cancer. Targeted treatment focused on HDAC4 may be a new strategy (Zang et al. 2022).
One report described a new non-coding RNA, circMAPK1, that is involved in the MAPK-signaling pathway, with its expression levels decreasing in gastric cancer. However, lower circMAPK1 expression levels are associated with lower survival rates in cancer patients. In gastric cancer, circMAPK1 plays an inhibitory role by encoding the MAPK1-109aa protein, specifically by inhibiting the phosphorylation of MAPK1 by competitively combining with MEK1 and then repressing the downstream MAPK pathway. CircMAPK1 is a good predictor of gastric cancer and provides a direction for treatment (Jiang et al. 2021).
There are many studies on gastric cancer cases involving human EGFR2. For example, in a clinical phase III study for advanced HER2 cancer, adding pembrolizumab to chemotherapy and trastuzumab could significantly reduce tumor size and improve the objective remission rates (Janjigian et al. 2021). However, because of the abnormal activation of HER2 and downstream signals, such as the amplification, mutation, or upregulation of HER2, KRAS, and AKT, the sensitivity and drug resistance of patients are insufficient. Poor patient response remains a clinical challenge (Shi et al. 2021). Inhibitors of the MAPK-signaling pathway have good efficacy when combined with other drugs, but some of these drugs alone have poor efficacy. For example, the MEK1 gene can have an activation mutation that causes gastric cancer. After treatment with trametinib alone, ERK1/2 is reactivated and the cancer cells become resistant to the drug. However, when used in combination with lapatinib, ERK1/2 activation was reversed and drug resistance was eliminated. Therefore, using drugs in combination is a possible treatment method to overcome drug resistance (Mizukami et al. 2015; Wang et al. 2021a, b).The anti-cancer intervention measures that affect the targeted MAPK-signaling pathway at different stages of clinical trials are shown in Table 1.
Natural product-targeted MAPK-signaling pathway for cancer prevention and treatment
Natural products play a significant role in treating diseases, especially various cancers and contagions, and as such have elicited the attention of many researchers (Atanasov et al. 2021). The chemical composition of products from different sources has become a promising method for preventing and treating cancer. A series of natural products are related to many signaling pathways and play an anti-tumor role. For example, sulforaphane, curcumin, quercetin, and resveratrol can affect the MAPK, PI3K/Akt, NF-κB, and other pathways to regulate the growth and proliferation of cancer cells (Shakeri et al. 2021). The following sections describe some of the natural active ingredients, summarize the relevant literature on natural products targeting the MAPK-signaling pathway in cancer, present preclinical studies from the last 5 years on different cancer types, and elucidate their mechanisms of action.
Flavonoids
Flavonoids belong to plant secondary metabolites, a class of compounds with C6–C3–C6 as their basic skeleton. In plants, this is mainly bound to sugars in the form of glycosides or carboglycosyl groups, and to a lesser extent in free form (Imran et al. 2019). Among the natural products that have been discovered so far, flavonoid components have been demonstrated to have significant anti-tumor activity by numerous studies. For example, eupatilin (Wang et al. 2018a, b, c, d) is a natural flavonoid. In esophageal tumor cells, eupatilin can inhibit ERK1/2 phosphorylation and growth of the esophageal tumor cell line TE1. In trials with the endometrial cancer cell lines Hec1A and KLE, eupatilin could upregulate ERK1/2 phosphorylation and inhibit tumor cell proliferation. Compared with cisplatin, its inhibitory effect was more effective and had fewer associated toxicity and side effects. According to reports, the activity of ERK1/2 protein is also related to apoptosis of oral squamous cell carcinoma cells. Hsieh et al. (2021) found that chrysosplenol D could inhibit the activation of key target proteins in the MAPK-signaling pathway, including ERK1/2, JNK, and p38, thereby enhancing the cleaved PARP activation and mediating the arrest and apoptosis of cancer cells. Flavonoids targeting the MAPK-signaling pathway and their regulatory mechanisms are shown in Table 2 and the flavonoid structures are shown in Fig. 3.
Phenols
Phenolic compounds are plant secondary metabolites that are widely found in nature. Phenolic compounds are structurally diverse and have antioxidant, anti-tumor, and antiviral effects (Almanza-Aguilera et al. 2023; Huminiecki 2022; Sorrenti et al. 2023). Phenolic compounds can regulate cancer cell apoptosis by modulating protein kinases in the MAPK-signaling pathway. Vanillin is a natural aromatic organic compound mainly found in plants, including vanilla pompon, vanilla planifolia, and vanilla tahitiensis. Vanillin can upregulate p38 phosphorylation in colorectal cancer cells and increase cancer cell apoptosis, with almost no treatment-related side effects (Li et al. 2021a, b, c). According to another study, vanillin can also inhibit the MAPKsignaling pathway, downregulate the phosphorylation of ERK, JNK, and p38 protein kinases, and reduce the number of cancer cells in colon tissues (Li et al. 2018a, b). Resveratrol is a natural phenolic compound with high anti-cancer activity and can be used to treat various cancers. According to a previous study, resveratrol can inhibit the ERK and p38 MAPK-signaling pathways by downregulating ERK and p38 phosphorylation in pancreatic cancer cells, reducing the proliferation and diffusion of cancer cells (Chen and Liu 2018). In addition, resveratrol prevents interleukin-6-induced gastric cancer metastasis by inhibiting RAF/MAPK pathway activation (Yang et al. 2018). The anti-cancer mechanisms of action of phenolic compounds are shown in Table 3 and the structures are illustrated in Fig. 4.
Terpenoids
Terpenes, most of which are polymers of isoprene and their derivatives, are a widely distributed class of natural products. They are classified based on the number of isoprene units in the molecular structure, including monoterpenes, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes. Each isoform has different biological activities, with some having anti-cancer effects (Wei et al. 2023; Zhang et al. 2023). Zerumbone (Lv et al. 2018) is a monocyclic sesquiterpenoid compound that is isolated from the root of Zingiber Zerumbet Smith. In HepG2 liver cancer cells, zerumbone can inhibit the metastasis and proliferation of hepatoma cells in a dose-dependent manner by downregulating ERK1/2 phosphorylation and upregulating p38 phosphorylation. Jalili-Nik et al. (2021) also showed that zerumbone can suppress glioblastoma multiform cancer cell metastasis by reducing ERK1/2 phosphorylation. Oridonin is a natural tetracyclic diterpenoid compound. Oridonin may induce oral cancer cell apoptosis by the ROS-mediated p38 and JNK pathways (Oh et al. 2018). Research in colon and pancreatic cancers suggested that oridonin can significantly upregulate p38 phosphorylation and participate in cancer cell apoptosis. When using a p38-specific inhibitor (SB203580), inhibition of p38 significantly attenuated the increase in p–p53 levels induced by oridonin. These results showed that oridonin can trigger p53 signaling in cancer cells via the p38 pathway and was directly involved in cancer cell apoptosis (Liu et al. 2018; Chen and Liu 2018). The mechanisms by which terpenoids modulate the MAPK-signaling pathway are shown in Table 4 and the structures are shown in Fig. 5.
Alkaloids
Alkaloids are a class of basic nitrogen-containing organic compounds found in nature, most of which have complex circular structures. Alkaloids are one of the clinically effective ingredients in the treatment of diseases (Gjorgieva Ackova et al. 2023; Guo et al. 2023). Wang et al. (2018a, b, c, d) found that evodiamine could upregulate the phosphorylation levels of p38 and JNK in glioblastoma multiforme cells, which led cancer cell apoptosis. Evodiamine also induced apoptosis in ovarian cancer cells by disrupting the mitochondrial membrane potential through activation of JNK and ERK. Zhao et al. (2021a, b) found that sophoridine significantly upregulated the phosphorylation levels of JNK, ERK, and p38, which in turn promoted macrophage M1 polarization, thereby inhibiting cancer cell growth. The anti-cancer mechanisms of action of alkaloids are shown in Table 5 and the structures are illustrated in Fig. 6.
Steroidal saponins
Steroids are a wide class of chemical compounds in nature, all of which have a cyclopentano-perhydrophenanthrene parent nucleus in their structures. Certain steroidal saponins are already being used in cancer treatment studies (Bouabdallah et al. 2023; Majnooni et al. 2023). For example, timosaponin AIII is a steroid saponin that can play an anti-cancer role in different cancers, especially breast cancer. Work in MDA-MB-2 and MCF231 breast cancer cell lines suggested that timosaponin AIII could trigger DNA damage by activating p38, then indirectly cause G2/M phase arrest that reduced cell survival (Zhang et al. 2020). The mechanisms by which steroids can regulate the MAPK-signaling pathway are shown in Table 6 and their structures are shown in Fig. 7.
Quinones
Quinones are natural organic compounds that contain unsaturated cyclic diketone structures and mainly include four types: benzoquinone, naphthoquinone, phenanthrenequinone, and anthraquinone. Wang et al. (2018a, b, c, d) found that juglone could induce the activation of the p38 and JNK MAPK-signaling pathways, which partly led to autophagy in HepG2 cells, G2/M cell cycle arrest, and increased apoptosis in hepatocellular carcinoma cells. In addition, Han et al. (2019) observed that shikonin not only enhanced the phosphorylation of ERK, JNK, and p38 in a time-dependent manner, but also co-induced apoptosis in SNU-407 colon cancer cells through ER stress response and mitochondrial pathways. The anti-cancer mechanisms of action of quinones are shown in Table 7 and the structures are illustrated in Fig. 8.
Discussion
Recently, research on the supercritical extract of rosemary was reported and registered in a clinical trial (NCT05080920). Work in non-small cell lung cancer showed that it is used in the clinic alongside standard cancer drugs, such as cisplatin and parrolizumab. Supercritical extract of rosemary can inhibit the MAPK-signaling pathway and enhance immune anti-cancer functions. Therefore, further research into its use as an adjuvant in the treatment of non-small cell lung cancer is warranted (Bouzas et al. 2022).
Kittiwattanokhun et al. (2021) revealed that S. alata extract reduces the expression levels of MMP-3 and MMP-1353 in cells and inhibits chondrosarcoma SW1353 cell migration. These observed effects are also connected with inhibition of the MAPK-signaling pathway.
Chen et al. (2018a, b) evaluated the effects of the combined use of antrodia cinnamomea and ginger on liver cancer cells, specifically the HepG2 and Huh-7 cell lines. The results showed that antrodia cinnamomea and ginger synergistically inhibited the MAPK-signaling pathway, significantly reducing ERK and p38 phosphorylation. Their effects when combined were better than those of using antrodia cinnamomea alone. This is possibly because the coculture of antrodia cinnamomea and ginger produced new triterpenoids, which changed the active components of the original plant. This concept may provide a valuable new direction for cancer treatment development.
Various internal and external factors contribute to cancer evolution. In different types of cancer, genetic changes, dysregulated signaling pathways, and loss of internal loop homeostasis all collectively support tumor growth. Alterations to components of the MAPK-signaling pathway are often present in various cancers. It is a crucial pathway for cancer cell drug resistance and proliferation, and is becoming a potential therapeutic target.
Various protein kinases in the MAPK-signaling pathway have been used as predictive biomarkers in preclinical studies of various cancers. Many researchers have also attempted to identify effective drugs to target this pathway, some of which are in clinical trials. However, drug resistance and side effects are major problems associated with these drugs. Because of the safety of natural products, the roles of their various chemical ingredients are gradually being explored. These include chrysophanol, rhein, brasinin, fargesin, and apocynin, which have been found to potentially target the MAPK pathway and are in the development stage of preclinical trials.
However, although many single-component anti-cancer mechanisms have been reported, there are few studies on the combined treatment of the chemical components of various natural products or their use in combination with clinical anticancer drugs. According to the existing in vitro experiments, the anti-cancer effects of using a combination therapy are significantly higher than those associated with a single drug. Second, because of the limitations of clinical research, there are few natural products in the clinical trial stage. However, some of these natural products are better than the existing synthetic MAPK inhibitors in terms of safety and effectiveness. Better treatment strategies may be obtained using them as an adjuvant or routine treatment for cancer. Exploring derivatives of these natural ingredients may also be a potential way to enhance the anti-cancer capacity of the original compounds.
Conclusion
The abnormal activation of specific proteins in the MAPK-signaling pathway is an important cause of various cancers. Therefore, therapeutically intervening in this signaling pathway may be an effective tumor treatment strategy. Considering the side effects of the existing MAPK inhibitors, exploring natural product compounds provides a new direction for cancer treatment. For example, vanillin, restoratrol, curcumin, oridonim, astragaloside IV, and ursolic acid have been extensively studied and found to have good anti-cancer effects. In the future, we should focus on the development of natural medicines, as well as their use in conjunction with existing medical technologies, to enhance cancer treatment approaches.
Data availability
Not applicable.
Abbreviations
- MAPKKK:
-
Mitogen-activated protein kinase kinase kinase
- MAPKK:
-
Mitogen-activated protein kinase kinase
- MAPK:
-
Mitogen-activated protein kinase
- ERK1/2:
-
Extracellular regulated kinase 1/2
- JNK:
-
C-Jun N-terminal kinase
- p38:
-
P38 mitogen-activated protein kinase
- ERK5:
-
Extracellular regulated kinase 5
- UCP1:
-
Uncoupling protein 1
- ROS:
-
Reactive oxygen species
- ECM:
-
Extracellular matrix
- TMEM2:
-
Transmembrane protein 2
- HA:
-
Hyaluronic
- ER:
-
Endoplasmic reticulum
- CD44:
-
Cluster of differentiation-44
- AICD:
-
Activation-induced cell death
- HSC:
-
Hemopoietic stem cell
- ISCs:
-
Intestinal stem cells
- EGFR:
-
Epidermal growth factor receptor
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TRPM2:
-
Transient receptor potential melastatin 2
- HDACs:
-
Histone deacetylases
References
Almanza-Aguilera E, Guiñón-Fort D, Perez-Cornago A et al (2023) Intake of the total, classes, and subclasses of (poly)phenols and risk of prostate cancer: a prospective analysis of the epic study. Cancers (basel). https://doi.org/10.3390/cancers15164067
Aranha ESP, Portilho AJS, Bentes de Sousa L et al (2021) 22β-hydroxytingenone induces apoptosis and suppresses invasiveness of melanoma cells by inhibiting mmp-9 activity and mapk signaling. J Ethnopharmacol 267:113605. https://doi.org/10.1016/j.jep.2020.113605
Ascierto PA, Stroyakovskiy D, Gogas H et al (2023) Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in braf(v600) mutation-positive advanced melanoma (imspire150): Second interim analysis of a multicentre, randomised, phase 3 study. Lancet Oncol 24(1):33–44. https://doi.org/10.1016/s1470-2045(22)00687-8
Ashraf A, Afroze SH, Yamauchi K et al (2018) Differential mechanism of action of 3,4’,7-o-trimethylquercetin in three types of ovarian cancer cells. Anticancer Res 38(9):5131–5137. https://doi.org/10.21873/anticanres.12835
Atanasov AG, Zotchev SB, Dirsch VM et al (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20(3):200–216. https://doi.org/10.1038/s41573-020-00114-z
Bae S, Lim JW, Kim H (2021) Β-carotene inhibits expression of matrix metalloproteinase-10 and invasion in helicobacter pylori-infected gastric epithelial cells. Molecules. https://doi.org/10.3390/molecules26061567
Bai X, Wei H, Liu W et al (2022) Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 71(12):2439–2450. https://doi.org/10.1136/gutjnl-2021-325021
Behr J, Prasse A, Kreuter M et al (2021) Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (relief): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 9(5):476–486. https://doi.org/10.1016/s2213-2600(20)30554-3
Bhattacharyya R, Gupta P, Bandyopadhyay SK et al (2018) Coralyne, a protoberberine alkaloid, causes robust photosenstization of cancer cells through atr-p38 mapk-bax and jak2-stat1-bax pathways. Chem Biol Interact 285:27–39. https://doi.org/10.1016/j.cbi.2018.02.032
Bouabdallah S, Al-Maktoum A, Amin A (2023) Steroidal saponins: naturally occurring compounds as inhibitors of the hallmarks of cancer. Cancers (basel). https://doi.org/10.3390/cancers15153900
Bouffet E, Geoerger B, Moertel C et al (2023) Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric braf v600-mutant low-grade glioma. J Clin Oncol 41(3):664–674. https://doi.org/10.1200/jco.22.01000
Bouzas A, Gómez de Cedrón M, Colmenarejo G et al (2022) Phenolic diterpenes from rosemary supercritical extract inhibit non-small cell lung cancer lipid metabolism and synergise with therapeutic drugs in the clinic. Front Oncol 12:1046369. https://doi.org/10.3389/fonc.2022.1046369
Brägelmann J, Lorenz C, Borchmann S et al (2021) Mapk-pathway inhibition mediates inflammatory reprogramming and sensitizes tumors to targeted activation of innate immunity sensor rig-i. Nat Commun 12(1):5505. https://doi.org/10.1038/s41467-021-25728-8
Bryant KL, Stalnecker CA, Zeitouni D et al (2019) Combination of erk and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med 25(4):628–640. https://doi.org/10.1038/s41591-019-0368-8
Cañellas-Socias A, Cortina C, Hernando-Momblona X et al (2022) Metastatic recurrence in colorectal cancer arises from residual emp1(+) cells. Nature 611(7936):603–613. https://doi.org/10.1038/s41586-022-05402-9
Cargnello M, Roux PP (2011) Activation and function of the mapks and their substrates, the mapk-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83. https://doi.org/10.1128/mmbr.00031-10
Chang WT, Bow YD, Fu PJ et al (2021) A marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ros and mapk pathways. Oxid Med Cell Longev 2021:7689045. https://doi.org/10.1155/2021/7689045
Chen H, Liu RH (2018) Potential mechanisms of action of dietary phytochemicals for cancer prevention by targeting cellular signaling transduction pathways. J Agric Food Chem 66(13):3260–3276. https://doi.org/10.1021/acs.jafc.7b04975
Chen J, Ma DN, Fang Y et al (2018a) Berberine hydrochloride counteracts enhanced il-8 expression induced by sn 38 in ags cells. J Asian Nat Prod Res 20(8):781–792. https://doi.org/10.1080/10286020.2017.1346629
Chen SY, Lee YR, Hsieh MC et al (2018b) Enhancing the anticancer activity of antrodia cinnamomea in hepatocellular carcinoma cells via cocultivation with ginger: The impact on cancer cell survival pathways. Front Pharmacol 9:780. https://doi.org/10.3389/fphar.2018.00780
Chen T, Yang P, Jia Y (2021) Molecular mechanisms of astragaloside-iv in cancer therapy (review). Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4846
Cheng Y, Sun F, Wang L et al (2020) Virus-induced p38 mapk activation facilitates viral infection. Theranostics 10(26):12223–12240. https://doi.org/10.7150/thno.50992
Cook SJ, Lochhead PA (2022) Erk5 signalling and resistance to erk1/2 pathway therapeutics: the path less travelled? Front Cell Dev Biol 10:839997. https://doi.org/10.3389/fcell.2022.839997
Corcoran RB, André T, Atreya CE et al (2018) Combined braf, egfr, and mek inhibition in patients with braf(v600e)-mutant colorectal cancer. Cancer Discov 8(4):428–443. https://doi.org/10.1158/2159-8290.Cd-17-1226
Cristea S, Coles GL, Hornburg D et al (2020) The mek5-erk5 kinase axis controls lipid metabolism in small-cell lung cancer. Cancer Res 80(6):1293–1303. https://doi.org/10.1158/0008-5472.Can-19-1027
Cui L, Bu W, Song J et al (2018) Apoptosis induction by alantolactone in breast cancer mda-mb-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Arch Pharm Res 41(3):299–313. https://doi.org/10.1007/s12272-017-0990-2
Das U, Manna K, Adhikary A et al (2019) Ferulic acid enhances the radiation sensitivity of lung and liver carcinoma cells by collapsing redox homeostasis: Mechanistic involvement of akt/p38 mapk signalling pathway. Free Radic Res 53(9–10):944–967. https://doi.org/10.1080/10715762.2019.1655559
Ding Y, Chen X, Wang B et al (2018) Quercetin suppresses the chymotrypsin-like activity of proteasome via inhibition of mek1/erk1/2 signaling pathway in hepatocellular carcinoma hepg2 cells. Can J Physiol Pharmacol 96(5):521–526. https://doi.org/10.1139/cjpp-2017-0655
Drosten M, Barbacid M (2020) Targeting the mapk pathway in kras-driven tumors. Cancer Cell 37(4):543–550. https://doi.org/10.1016/j.ccell.2020.03.013
Elez E, Ros J, Fernández J et al (2022) Rnf43 mutations predict response to anti-braf/egfr combinatory therapies in braf(v600e) metastatic colorectal cancer. Nat Med 28(10):2162–2170. https://doi.org/10.1038/s41591-022-01976-z
El-Kott AF, ElBealy ER, Alshehri AS et al (2021) Salidroside induces cell apoptosis and inhibits the invasiveness of ht29 colorectal cells by regulating protein kinase r, nf-κb and stat3. Cancer Biomark 31(1):13–25. https://doi.org/10.3233/cbm-203257
Elwakeel E, Brüggemann M, Wagih J et al (2022) Disruption of prostaglandin e2 signaling in cancer-associated fibroblasts limits mammary carcinoma growth but promotes metastasis. Cancer Res 82(7):1380–1395. https://doi.org/10.1158/0008-5472.Can-21-2116
Fang XY, Zhang H, Zhao L et al (2018) A new xanthatin analogue 1β-hydroxyl-5α-chloro-8-epi-xanthatin induces apoptosis through ros-mediated erk/p38 mapk activation and jak2/stat3 inhibition in human hepatocellular carcinoma. Biochimie 152:43–52. https://doi.org/10.1016/j.biochi.2018.06.018
Feng P, Yang Q, Luo L et al (2021) The kinase pdk1 regulates regulatory t cell survival via controlling redox homeostasis. Theranostics 11(19):9503–9518. https://doi.org/10.7150/thno.63992
Geller S, Arribat Y, Netzahualcoyotzi C et al (2019) Tanycytes regulate lipid homeostasis by sensing free fatty acids and signaling to key hypothalamic neuronal populations via fgf21 secretion. Cell Metab 30(4):833-844.e837. https://doi.org/10.1016/j.cmet.2019.08.004
Gershenson DM, Miller A, Brady WE et al (2022) Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (gog 281/logs): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 399(10324):541–553. https://doi.org/10.1016/s0140-6736(21)02175-9
Gill BS, Navgeet MR et al (2018) Ganoderic acid, lanostanoid triterpene: a key player in apoptosis. Invest New Drugs 36(1):136–143. https://doi.org/10.1007/s10637-017-0526-0
Gjorgieva Ackova D, Maksimova V, Smilkov K et al (2023) Alkaloids as natural nrf2 inhibitors: chemoprevention and cytotoxic action in cancer. Pharmaceuticals (basel). https://doi.org/10.3390/ph16060850
Gross AM, Wolters PL, Dombi E et al (2020) Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382(15):1430–1442. https://doi.org/10.1056/NEJMoa1912735
Grothey A, Fakih M, Tabernero J (2021) Management of braf-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol 32(8):959–967. https://doi.org/10.1016/j.annonc.2021.03.206
Guo X, Ding X (2018) Dioscin suppresses the viability of ovarian cancer cells by regulating the vegfr2 and pi3k/akt/mapk signaling pathways. Oncol Lett 15(6):9537–9542. https://doi.org/10.3892/ol.2018.8454
Guo X, Ding X, Dong J (2022) Dichotomy of the bsl phosphatase signaling spatially regulates mapk components in stomatal fate determination. Nat Commun 13(1):2438. https://doi.org/10.1038/s41467-022-30254-2
Guo C, Zhang L, Zhao M et al (2023) Targeting lipid metabolism with natural products: a novel strategy for gastrointestinal cancer therapy. Phytother Res 37(5):2036–2050. https://doi.org/10.1002/ptr.7735
Han X, Kang KA, Piao MJ et al (2019) Shikonin exerts cytotoxic effects in human colon cancers by inducing apoptotic cell death via the endoplasmic reticulum and mitochondria-mediated pathways. Biomol Ther (seoul) 27(1):41–47. https://doi.org/10.4062/biomolther.2018.047
Harbeck N, Penault-Llorca F, Cortes J et al (2019) Breast cancer. Nat Rev Dis Primers 5(1):66. https://doi.org/10.1038/s41572-019-0111-2
He Y, Meng X (2020) Mapk signaling: emerging roles in lateral root formation. Trends Plant Sci 25(2):126–129. https://doi.org/10.1016/j.tplants.2019.11.006
Herman JA, Romain RR, Hoellerbauer P et al (2022) Hyper-active ras/mapk introduces cancer-specific mitotic vulnerabilities. Proc Natl Acad Sci USA 119(41):e2208255119. https://doi.org/10.1073/pnas.2208255119
Hsieh MJ, Lin CC, Lo YS et al (2021) Chrysosplenol d triggers apoptosis through heme oxygenase-1 and mitogen-activated protein kinase signaling in oral squamous cell carcinoma. Cancers (basel). https://doi.org/10.3390/cancers13174327
Hu Y, Li X, Lin L et al (2018) Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol Rep 39(4):1731–1738. https://doi.org/10.3892/or.2018.6234
Huang KJ, Kuo CH, Chen SH et al (2018) Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med 22(3):1894–1908. https://doi.org/10.1111/jcmm.13474
Huang X, Liang C, Yang H et al (2021a) Curcumin induces apoptosis and inhibits the growth of adrenocortical carcinoma: identification of potential candidate genes and pathways by transcriptome analysis. Oncol Lett 21(6):476. https://doi.org/10.3892/ol.2021.12737
Huang Y, Zhang X, Pang Q et al (2021b) Proliferation of mda-mb-231 can be suppressed by dimeric-epigallocatechin gallate through competitive inhibition of amphiregulin-epidermal growth factor receptor signaling. Anticancer Drugs 32(6):647–656. https://doi.org/10.1097/cad.0000000000001038
Huminiecki L (2022) Evidence for multilevel chemopreventive activities of natural phenols from functional genomic studies of curcumin, resveratrol, genistein, quercetin, and luteolin. Int J Mol Sci. https://doi.org/10.3390/ijms232314957
Imran M, Rauf A, Abu-Izneid T et al (2019) Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother 112:108612. https://doi.org/10.1016/j.biopha.2019.108612
Jalili-Nik M, Afshari AR, Sabri H et al (2021) Zerumbone, a ginger sesquiterpene, inhibits migration, invasion, and metastatic behavior of human malignant glioblastoma multiforme in vitro. BioFactors 47(5):729–739. https://doi.org/10.1002/biof.1756
Janjigian YY, Kawazoe A, Yañez P et al (2021) The keynote-811 trial of dual pd-1 and her2 blockade in her2-positive gastric cancer. Nature 600(7890):727–730. https://doi.org/10.1038/s41586-021-04161-3
Jiang T, Xia Y, Lv J et al (2021) A novel protein encoded by circmapk1 inhibits progression of gastric cancer by suppressing activation of mapk signaling. Mol Cancer 20(1):66. https://doi.org/10.1186/s12943-021-01358-y
Johdi NA, Sukor NF (2020) Colorectal cancer immunotherapy: Options and strategies. Front Immunol 11:1624. https://doi.org/10.3389/fimmu.2020.01624
Johnson RM, Qu X, Lin CF et al (2022) Arid1a mutations confer intrinsic and acquired resistance to cetuximab treatment in colorectal cancer. Nat Commun 13(1):5478. https://doi.org/10.1038/s41467-022-33172-5
Joshi SS, Badgwell BD (2021) Current treatment and recent progress in gastric cancer. CA Cancer J Clin 71(3):264–279. https://doi.org/10.3322/caac.21657
Jung SH, Park SS, Lim JY et al (2022) Single-cell analysis of multiple myelomas refines the molecular features of bortezomib treatment responsiveness. Exp Mol Med 54(11):1967–1978. https://doi.org/10.1038/s12276-022-00884-z
Kang HM, Park BS, Kang HK et al (2018) Delphinidin induces apoptosis and inhibits epithelial-to-mesenchymal transition via the erk/p38 mapk-signaling pathway in human osteosarcoma cell lines. Environ Toxicol 33(6):640–649. https://doi.org/10.1002/tox.22548
Keinan O, Valentine JM, Xiao H et al (2021) Glycogen metabolism links glucose homeostasis to thermogenesis in adipocytes. Nature 599(7884):296–301. https://doi.org/10.1038/s41586-021-04019-8
Kent OA, Saha M, Coyaud E et al (2020) Haploinsufficiency of rreb1 causes a noonan-like rasopathy via epigenetic reprogramming of ras-mapk pathway genes. Nat Commun 11(1):4673. https://doi.org/10.1038/s41467-020-18483-9
Kim DH, Shin EA, Kim B et al (2018) Reactive oxygen species-mediated phosphorylation of p38 signaling is critically involved in apoptotic effect of tanshinone i in colon cancer cells. Phytother Res 32(10):1975–1982. https://doi.org/10.1002/ptr.6126
Kittiwattanokhun A, Samosorn S, Innajak S et al (2021) Inhibitory effects on chondrosarcoma cell metastasis by senna alata extract. Biomed Pharmacother 137:111337. https://doi.org/10.1016/j.biopha.2021.111337
Klein AP (2021) Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol 18(7):493–502. https://doi.org/10.1038/s41575-021-00457-x
Kwak AW, Lee MJ, Lee MH et al (2021) The 3-deoxysappanchalcone induces ros-mediated apoptosis and cell cycle arrest via jnk/p38 mapks signaling pathway in human esophageal cancer cells. Phytomedicine 86:153564. https://doi.org/10.1016/j.phymed.2021.153564
Lee S, Rauch J, Kolch W (2020) Targeting mapk signaling in cancer: Mechanisms of drug resistance and sensitivity. Int J Mol Sci. https://doi.org/10.3390/ijms21031102
Li JM, Lee YC, Li CC et al (2018a) Vanillin-ameliorated development of azoxymethane/dextran sodium sulfate-induced murine colorectal cancer: The involvement of proteasome/nuclear factor-κb/mitogen-activated protein kinase pathways. J Agric Food Chem 66(22):5563–5573. https://doi.org/10.1021/acs.jafc.8b01582
Li S, Cheng B, Hou L et al (2018b) Dioscin inhibits colon cancer cells’ growth by reactive oxygen species-mediated mitochondrial dysfunction and p38 and jnk pathways. Anticancer Drugs 29(3):234–242. https://doi.org/10.1097/cad.0000000000000590
Li G, Kong B, Tong Q et al (2021a) Vanillin downregulates nnmt and attenuates nnmt-related resistance to 5-fluorouracil via ros-induced cell apoptosis in colorectal cancer cells. Oncol Rep. https://doi.org/10.3892/or.2021.8061
Li L, Wang J, Feng L et al (2021b) Rubioncolin c, a natural naphthohydroquinone dimer isolated from rubia yunnanensis, inhibits the proliferation and metastasis by inducing ros-mediated apoptotic and autophagic cell death in triple-negative breast cancer cells. J Ethnopharmacol 277:114184. https://doi.org/10.1016/j.jep.2021.114184
Li S, Fang J, Si T et al (2021c) Salvianolic acid a inhibits the growth of diffuse large b-cell lymphoma through mapk pathways. Exp Hematol 94:60-68.e62. https://doi.org/10.1016/j.exphem.2020.11.007
Li GN, Zhao XJ, Wang Z et al (2022) Elaiophylin triggers paraptosis and preferentially kills ovarian cancer drug-resistant cells by inducing mapk hyperactivation. Signal Transduct Target Ther 7(1):317. https://doi.org/10.1038/s41392-022-01131-7
Liang Y, Zhang T, Ren L et al (2021) Cucurbitacin iib induces apoptosis and cell cycle arrest through regulating egfr/mapk pathway. Environ Toxicol Pharmacol 81:103542. https://doi.org/10.1016/j.etap.2020.103542
Liao CC, Chen SC, Huang HP et al (2018) Gallic acid inhibits bladder cancer cell proliferation and migration via regulating fatty acid synthase (fas). J Food Drug Anal 26(2):620–627. https://doi.org/10.1016/j.jfda.2017.06.006
Lim W, An Y, Yang C et al (2018a) Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. J Cell Biochem 119(12):10216–10227. https://doi.org/10.1002/jcb.27363
Lim W, Ryu S, Bazer FW et al (2018b) Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J Cell Physiol 233(4):3129–3140. https://doi.org/10.1002/jcp.26150
Lim W, Ham J, Bazer FW et al (2019) Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J Cell Physiol 234(2):1803–1815. https://doi.org/10.1002/jcp.27054
Lin R, Bao X, Wang H et al (2021) Trpm2 promotes pancreatic cancer by pkc/mapk pathway. Cell Death Dis 12(6):585. https://doi.org/10.1038/s41419-021-03856-9
Liu RX, Ma Y, Hu XL et al (2018) Anticancer effects of oridonin on colon cancer are mediated via bmp7/p38 mapk/p53 signaling. Int J Oncol 53(5):2091–2101. https://doi.org/10.3892/ijo.2018.4527
Liu C, Huang Y, Qin T et al (2022) Azd5153 reverses palbociclib resistance in ovarian cancer by inhibiting cell cycle-related proteins and the mapk/pi3k-akt pathway. Cancer Lett 528:31–44. https://doi.org/10.1016/j.canlet.2021.12.021
Lv T, Zhang W, Han X (2018) Zerumbone suppresses the potential of growth and metastasis in hepatoma hepg2 cells via the mapk signaling pathway. Oncol Lett 15(5):7603–7610. https://doi.org/10.3892/ol.2018.8335
Lv F, Du Q, Li L et al (2021) Eriodictyol inhibits glioblastoma migration and invasion by reversing emt via downregulation of the p38 mapk/gsk-3β/zeb1 pathway. Eur J Pharmacol 900:174069. https://doi.org/10.1016/j.ejphar.2021.174069
Ma L, Meng Y, Tu C et al (2018a) A cardiac glycoside htf-1 isolated from helleborus thibetanus franch displays potent in vitro anti-cancer activity via caspase-9, mapk and pi3k-akt-mtor pathways. Eur J Med Chem 158:743–752. https://doi.org/10.1016/j.ejmech.2018.09.019
Ma YL, Chen F, Shi J (2018b) Rhein inhibits malignant phenotypes of human renal cell carcinoma by impacting on mapk/nf-κb signaling pathways. Onco Targets Ther 11:1385–1394. https://doi.org/10.2147/ott.S153798
Majnooni MB, Fakhri S, Ghanadian SM et al (2023) Inhibiting angiogenesis by anti-cancer saponins: from phytochemistry to cellular signaling pathways. Metabolites. https://doi.org/10.3390/metabo13030323
Mao JJ, Pillai GG, Andrade CJ et al (2022) Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin 72(2):144–164. https://doi.org/10.3322/caac.21706
Meng LQ, Liu C, Luo YH et al (2018) Quinalizarin exerts an anti-tumour effect on lung cancer a549 cells by modulating the akt, mapk, stat3 and p53 signalling pathways. Mol Med Rep 17(2):2626–2634. https://doi.org/10.3892/mmr.2017.8110
Mizrahi JD, Surana R, Valle JW et al (2020) Pancreatic cancer. Lancet 395(10242):2008–2020. https://doi.org/10.1016/s0140-6736(20)30974-0
Mizukami T, Togashi Y, Sogabe S et al (2015) Egfr and her2 signals play a salvage role in mek1-mutated gastric cancer after mek inhibition. Int J Oncol 47(2):499–505. https://doi.org/10.3892/ijo.2015.3050
Mohan CD, Liew YY, Jung YY et al (2021) Brucein d modulates mapk signaling cascade to exert multi-faceted anti-neoplastic actions against breast cancer cells. Biochimie 182:140–151. https://doi.org/10.1016/j.biochi.2021.01.009
Moore AR, Rosenberg SC, McCormick F et al (2020) Ras-targeted therapies: Is the undruggable drugged? Nat Rev Drug Discov 19(8):533–552. https://doi.org/10.1038/s41573-020-0068-6
Oh HN, Seo JH, Lee MH et al (2018) Oridonin induces apoptosis in oral squamous cell carcinoma probably through the generation of reactive oxygen species and the p38/jnk mapk pathway. Int J Oncol 52(5):1749–1759. https://doi.org/10.3892/ijo.2018.4319
Ojulari OV, Chae JB, Lee SG et al (2021) Apoptotic effect of jaceosidin on mcf-7 human breast cancer cells through modulation of erk and p38 mapk pathways. Nat Prod Res 35(24):6049–6053. https://doi.org/10.1080/14786419.2020.1817917
Ouyang J, Liu Z, Yuan X et al (2021) Lncrna prncr1 promotes breast cancer proliferation and inhibits apoptosis by modulating microrna-377/ccnd2/mek/mapk axis. Arch Med Res 52(5):471–482. https://doi.org/10.1016/j.arcmed.2021.01.007
Pan H, Lu LY, Wang XQ et al (2018) Gambogic acid induces cell apoptosis and inhibits mapk pathway in pten(-/-)/p53(-/-) prostate cancer cells in vitro and ex vivo. Chin J Integr Med 24(2):109–116. https://doi.org/10.1007/s11655-017-2410-3
Park HB, Baek KH (2022) E3 ligases and deubiquitinating enzymes regulating the mapk signaling pathway in cancers. Biochim Biophys Acta Rev Cancer 1877(3):188736. https://doi.org/10.1016/j.bbcan.2022.188736
Park H, Park S, Bazer FW et al (2018a) Myricetin treatment induces apoptosis in canine osteosarcoma cells by inducing DNA fragmentation, disrupting redox homeostasis, and mediating loss of mitochondrial membrane potential. J Cell Physiol 233(9):7457–7466. https://doi.org/10.1002/jcp.26598
Park S, Lim W, Jeong W et al (2018b) Sideroxylin (callistemon lanceolatus) suppressed cell proliferation and increased apoptosis in ovarian cancer cells accompanied by mitochondrial dysfunction, the generation of reactive oxygen species, and an increase of lipid peroxidation. J Cell Physiol 233(11):8597–8604. https://doi.org/10.1002/jcp.26540
Parseghian CM, Sun R, Woods M et al (2023) Resistance mechanisms to anti-epidermal growth factor receptor therapy in ras/raf wild-type colorectal cancer vary by regimen and line of therapy. J Clin Oncol 41(3):460–471. https://doi.org/10.1200/jco.22.01423
Pashayan N, Antoniou AC, Ivanus U et al (2020) Personalized early detection and prevention of breast cancer: envision consensus statement. Nat Rev Clin Oncol 17(11):687–705. https://doi.org/10.1038/s41571-020-0388-9
Pedrosa R, Mustafa DA, Soffietti R et al (2018) Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro Oncol 20(11):1439–1449. https://doi.org/10.1093/neuonc/noy044
Ponsioen B, Post JB, Buissant des Amorie JR et al (2021) Quantifying single-cell erk dynamics in colorectal cancer organoids reveals egfr as an amplifier of oncogenic mapk pathway signalling. Nat Cell Biol 23(4):377–390. https://doi.org/10.1038/s41556-021-00654-5
Qi SZ, Zhang XX, Jin Y et al (2021) Phenylpropanoid-conjugated pentacyclic triterpenoids from the whole plants of leptopus lolonum induced cell apoptosis via mapk and akt pathways in human hepatocellular carcinoma cells. Bioorg Chem 111:104886. https://doi.org/10.1016/j.bioorg.2021.104886
Qin G, Li P, Xue Z (2018) Triptolide induces protective autophagy and apoptosis in human cervical cancer cells by downregulating akt/mtor activation. Oncol Lett 16(3):3929–3934. https://doi.org/10.3892/ol.2018.9074
Raina R, Pramodh S, Rais N et al (2021) Luteolin inhibits proliferation, triggers apoptosis and modulates akt/mtor and map kinase pathways in hela cells. Oncol Lett 21(3):192. https://doi.org/10.3892/ol.2021.12452
Rajedadram A, Pin KY, Ling SK et al (2021) Hydroxychavicol, a polyphenol from piper betle leaf extract, induces cell cycle arrest and apoptosis in tp53-resistant ht-29 colon cancer cells. J Zhejiang Univ Sci B 22(2):112–122. https://doi.org/10.1631/jzus.B2000446
Ravichandran M, Hu J, Cai C et al (2022) Coordinated transcriptional and catabolic programs support iron-dependent adaptation to ras-mapk pathway inhibition in pancreatic cancer. Cancer Discov 12(9):2198–2219. https://doi.org/10.1158/2159-8290.Cd-22-0044
Razavi P, Chang MT, Xu G et al (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34(3):427-438.e426. https://doi.org/10.1016/j.ccell.2018.08.008
Ren B, Cui M, Yang G et al (2018a) Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer 17(1):108. https://doi.org/10.1186/s12943-018-0858-1
Ren X, Zhao B, Chang H et al (2018b) Paclitaxel suppresses proliferation and induces apoptosis through regulation of ros and the akt/mapk signaling pathway in canine mammary gland tumor cells. Mol Med Rep 17(6):8289–8299. https://doi.org/10.3892/mmr.2018.8868
Rock CL, Thomson CA, Sullivan KR et al (2022) American cancer society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72(3):230–262. https://doi.org/10.3322/caac.21719
Rodenak-Kladniew B, Castro A, Stärkel P et al (2018) Linalool induces cell cycle arrest and apoptosis in hepg2 cells through oxidative stress generation and modulation of ras/mapk and akt/mtor pathways. Life Sci 199:48–59. https://doi.org/10.1016/j.lfs.2018.03.006
Sang J, Li W, Diao HJ et al (2021) Jolkinolide b targets thioredoxin and glutathione systems to induce ros-mediated paraptosis and apoptosis in bladder cancer cells. Cancer Lett 509:13–25. https://doi.org/10.1016/j.canlet.2021.03.030
Schinzel RT, Higuchi-Sanabria R, Shalem O et al (2019) The hyaluronidase, tmem2, promotes er homeostasis and longevity independent of the upr(er). Cell 179(6):1306-1318.e1318. https://doi.org/10.1016/j.cell.2019.10.018
Shakeri A, Ghanbari M, Tasbandi A et al (2021) Regulation of microrna-21 expression by natural products in cancer. Phytother Res 35(7):3732–3746. https://doi.org/10.1002/ptr.7069
Shao J, Wang C, Li L et al (2018) Luteoloside inhibits proliferation and promotes intrinsic and extrinsic pathway-mediated apoptosis involving mapk and mtor signaling pathways in human cervical cancer cells. Int J Mol Sci. https://doi.org/10.3390/ijms19061664
Shi L, Qin H, Jin X et al (2018) The natural phenolic peperobtusin a induces apoptosis of lymphoma u937 cells via the caspase dependent and p38 mapk signaling pathways. Biomed Pharmacother 102:772–781. https://doi.org/10.1016/j.biopha.2018.03.141
Shi W, Zhang G, Ma Z et al (2021) Hyperactivation of her2-shcbp1-plk1 axis promotes tumor cell mitosis and impairs trastuzumab sensitivity to gastric cancer. Nat Commun 12(1):2812. https://doi.org/10.1038/s41467-021-23053-8
Si L, Yang R, Lin R et al (2018) Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of jnk/p38 mapk-mediated intrinsic apoptotic pathway. Biosci Rep. https://doi.org/10.1042/bsr20180503
Siegel RL, Miller KD, Fuchs HE et al (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Simula L, Corrado M, Accordi B et al (2020) Jnk1 and erk1/2 modulate lymphocyte homeostasis via bim and drp1 upon aicd induction. Cell Death Differ 27(10):2749–2767. https://doi.org/10.1038/s41418-020-0540-1
Smyth EC, Nilsson M, Grabsch HI et al (2020) Gastric cancer. Lancet 396(10251):635–648. https://doi.org/10.1016/s0140-6736(20)31288-5
Sorrenti V, Buriani A, Fortinguerra S et al (2023) Cell survival, death, and proliferation in senescent and cancer cells: the role of (poly)phenols. Adv Nutr 14(5):1111–1130. https://doi.org/10.1016/j.advnut.2023.05.014
Stramucci L, Pranteda A, Stravato A et al (2019) Mkk3 sustains cell proliferation and survival through p38delta mapk activation in colorectal cancer. Cell Death Dis 10(11):842. https://doi.org/10.1038/s41419-019-2083-2
Su MQ, Zhou YR, Rao X et al (2018) Baicalein induces the apoptosis of hct116 human colon cancer cells via the upregulation of depp/gadd45a and activation of mapks. Int J Oncol 53(2):750–760. https://doi.org/10.3892/ijo.2018.4402
Su Q, Wang J, Wu Q et al (2021) Sanguinarine combats hypoxia-induced activation of ephb4 and hif-1α pathways in breast cancer. Phytomedicine 84:153503. https://doi.org/10.1016/j.phymed.2021.153503
Sui X, Kong N, Ye L et al (2014) P38 and jnk mapk pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344(2):174–179. https://doi.org/10.1016/j.canlet.2013.11.019
Sun Y, Lan M, Chen X et al (2018) Anti-invasion and anti-metastasis effects of valjatrate e via reduction of matrix metalloproteinases expression and suppression of mapk/erk signaling pathway. Biomed Pharmacother 104:817–824. https://doi.org/10.1016/j.biopha.2018.04.136
Sun H, Ou B, Zhao S et al (2019) Usp11 promotes growth and metastasis of colorectal cancer via ppp1ca-mediated activation of erk/mapk signaling pathway. EBioMedicine 48:236–247. https://doi.org/10.1016/j.ebiom.2019.08.061
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Tadokoro Y, Hoshii T, Yamazaki S et al (2018) Spred1 safeguards hematopoietic homeostasis against diet-induced systemic stress. Cell Stem Cell 22(5):713-725.e718. https://doi.org/10.1016/j.stem.2018.04.002
Tubita A, Lombardi Z, Tusa I et al (2022) Inhibition of erk5 elicits cellular senescence in melanoma via the cyclin-dependent kinase inhibitor p21. Cancer Res 82(3):447–457. https://doi.org/10.1158/0008-5472.Can-21-0993
Tungalag T, Yang DK (2021) Sinapic acid protects sh-sy5y human neuroblastoma cells against 6-hydroxydopamine-induced neurotoxicity. Biomedicines. https://doi.org/10.3390/biomedicines9030295
Ullah R, Yin Q, Snell AH et al (2022) Raf-mek-erk pathway in cancer evolution and treatment. Semin Cancer Biol 85:123–154. https://doi.org/10.1016/j.semcancer.2021.05.010
Vincent A, Herman J, Schulick R et al (2011) Pancreatic cancer. Lancet 378(9791):607–620. https://doi.org/10.1016/s0140-6736(10)62307-0
Wang JN, Zhang ZR, Che Y et al (2018a) Acetyl-macrocalin b, an ent-kaurane diterpenoid, initiates apoptosis through the ros-p38-caspase 9-dependent pathway and induces g2/m phase arrest via the chk1/2-cdc25c-cdc2/cyclin b axis in non-small cell lung cancer. Cancer Biol Ther 19(7):609–621. https://doi.org/10.1080/15384047.2018.1449613
Wang P, Gao C, Wang W et al (2018b) Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem Toxicol 116(Pt B):40–50. https://doi.org/10.1016/j.fct.2018.04.004
Wang R, Deng D, Shao N et al (2018c) Evodiamine activates cellular apoptosis through suppressing pi3k/akt and activating mapk in glioma. Onco Targets Ther 11:1183–1192. https://doi.org/10.2147/ott.S155275
Wang X, Zhu Y, Zhu L et al (2018d) Eupatilin inhibits the proliferation of human esophageal cancer te1 cells by targeting the akt-gsk3β and mapk/erk signaling cascades. Oncol Rep 39(6):2942–2950. https://doi.org/10.3892/or.2018.6390
Wang H, Lv Q, Xu Y et al (2019) An integrative pharmacogenomics analysis identifies therapeutic targets in kras-mutant lung cancer. EBioMedicine 49:106–117. https://doi.org/10.1016/j.ebiom.2019.10.012
Wang H, Zhang K, Liu J et al (2021a) Curcumin regulates cancer progression: Focus on ncrnas and molecular signaling pathways. Front Oncol 11:660712. https://doi.org/10.3389/fonc.2021.660712
Wang X, Dai C, Yin Y et al (2021b) Blocking the jak2/stat3 and erk pathways suppresses the proliferation of gastrointestinal cancers by inducing apoptosis. J Zhejiang Univ Sci B 22(6):492–503. https://doi.org/10.1631/jzus.B2000842
Wei Z, Chen J, Zuo F et al (2023) Traditional chinese medicine has great potential as candidate drugs for lung cancer: a review. J Ethnopharmacol 300:115748. https://doi.org/10.1016/j.jep.2022.115748
Wen S, Hou Y, Fu L et al (2019) Cancer-associated fibroblast (caf)-derived il32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 mapk signalling. Cancer Lett 442:320–332. https://doi.org/10.1016/j.canlet.2018.10.015
Wu S, Chen M, Huang J et al (2021) Orai2 promotes gastric cancer tumorigenicity and metastasis through pi3k/akt signaling and mapk-dependent focal adhesion disassembly. Cancer Res 81(4):986–1000. https://doi.org/10.1158/0008-5472.Can-20-0049
Xia Y, Yuan M, Li S et al (2018) Apigenin suppresses the il-1β-induced expression of the urokinase-type plasminogen activator receptor by inhibiting mapk-mediated ap-1 and nf-κb signaling in human bladder cancer t24 cells. J Agric Food Chem 66(29):7663–7673. https://doi.org/10.1021/acs.jafc.8b02351
Xie SJ, Li JH, Chen HF et al (2018) Inhibition of the jnk/mapk signaling pathway by myogenesis-associated mirnas is required for skeletal muscle development. Cell Death Differ 25(9):1581–1597. https://doi.org/10.1038/s41418-018-0063-1
Xu X, Zhang M, Xu F et al (2020) Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol Cancer 19(1):165. https://doi.org/10.1186/s12943-020-01276-5
Xu Y, Huang Y, Chen Y et al (2021) Grape seed proanthocyanidins play the roles of radioprotection on normal lung and radiosensitization on lung cancer via differential regulation of the mapk signaling pathway. J Cancer 12(10):2844–2854. https://doi.org/10.7150/jca.49987
Yaeger R, Corcoran RB (2019) Targeting alterations in the raf-mek pathway. Cancer Discov 9(3):329–341. https://doi.org/10.1158/2159-8290.Cd-18-1321
Yamagata K, Izawa Y, Onodera D et al (2018) Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in a549 human lung cancer cells. Mol Cell Biochem 441(1–2):9–19. https://doi.org/10.1007/s11010-017-3171-1
Yang T, Zhang J, Zhou J et al (2018) Resveratrol inhibits interleukin-6 induced invasion of human gastric cancer cells. Biomed Pharmacother 99:766–773. https://doi.org/10.1016/j.biopha.2018.01.153
Yang Z, Zhang Q, Yu L et al (2021) The signaling pathways and targets of traditional chinese medicine and natural medicine in triple-negative breast cancer. J Ethnopharmacol 264:113249. https://doi.org/10.1016/j.jep.2020.113249
Yuan J, Dong X, Yap J et al (2020) The mapk and ampk signalings: Interplay and implication in targeted cancer therapy. J Hematol Oncol 13(1):113. https://doi.org/10.1186/s13045-020-00949-4
Zaman K, Winterhalder R, Mamot C et al (2015) Fulvestrant with or without selumetinib, a mek 1/2 inhibitor, in breast cancer progressing after aromatase inhibitor therapy: a multicentre randomised placebo-controlled double-blind phase ii trial, sakk 21/08. Eur J Cancer 51(10):1212–1220. https://doi.org/10.1016/j.ejca.2015.03.016
Zang WJ, Hu YL, Qian CY et al (2022) Hdac4 promotes the growth and metastasis of gastric cancer via autophagic degradation of mekk3. Br J Cancer 127(2):237–248. https://doi.org/10.1038/s41416-022-01805-7
Zeng GZ, Wang Z, Zhao LM et al (2018a) Nf-κb and jnk mediated apoptosis and g(0)/g(1) arrest of hela cells induced by rubiarbonol g, an arborinane-type triterpenoid from rubia yunnanensis. J Ethnopharmacol 220:220–227. https://doi.org/10.1016/j.jep.2017.10.026
Zeng Z, Zhang H, Wang X et al (2018b) Salvianolic acid b suppresses cell proliferation and induces apoptosis in osteosarcoma through p38-mediated reactive oxygen species generation. Oncol Lett 15(2):2679–2685. https://doi.org/10.3892/ol.2017.7609
Zhang J, Song Y, Liang Y et al (2019a) Cucurbitacin iia interferes with egfr-mapk signaling pathway leads to proliferation inhibition in a549 cells. Food Chem Toxicol 132:110654. https://doi.org/10.1016/j.fct.2019.110654
Zhang P, Holowatyj AN, Ulrich CM et al (2019b) Tumor suppressive autophagy in intestinal stem cells controls gut homeostasis. Autophagy 15(9):1668–1670. https://doi.org/10.1080/15548627.2019.1633863
Zhang M, Qu J, Gao Z et al (2020) Timosaponin aiii induces g2/m arrest and apoptosis in breast cancer by activating the atm/chk2 and p38 mapk signaling pathways. Front Pharmacol 11:601468. https://doi.org/10.3389/fphar.2020.601468
Zhang F, Ni ZJ, Ye L et al (2021a) Asparanin a inhibits cell migration and invasion in human endometrial cancer via ras/erk/mapk pathway. Food Chem Toxicol 150:112036. https://doi.org/10.1016/j.fct.2021.112036
Zhang L, Yang C, Huang Y et al (2021b) Cardamonin inhibits the growth of human osteosarcoma cells through activating p38 and jnk signaling pathway. Biomed Pharmacother 134:111155. https://doi.org/10.1016/j.biopha.2020.111155
Zhang Y, Zhang JQ, Zhang T et al (2021c) Calycosin induces gastric cancer cell apoptosis via the ros-mediated mapk/stat3/nf-κb pathway. Onco Targets Ther 14:2505–2517. https://doi.org/10.2147/ott.S292388
Zhang P, Guan H, Yuan S et al (2022) Targeting myeloid derived suppressor cells reverts immune suppression and sensitizes braf-mutant papillary thyroid cancer to mapk inhibitors. Nat Commun 13(1):1588. https://doi.org/10.1038/s41467-022-29000-5
Zhang Y, Peng F, Yu C (2023) Therapeutic potential of curcuma oil and its terpenoids in gynecological cancers. Biomed Pharmacother 157:114016. https://doi.org/10.1016/j.biopha.2022.114016
Zhao S, Jiang Y, Zhao J et al (2018) Quercetin-3-methyl ether inhibits esophageal carcinogenesis by targeting the akt/mtor/p70s6k and mapk pathways. Mol Carcinog 57(11):1540–1552. https://doi.org/10.1002/mc.22876
Zhao B, Hui X, Zeng H et al (2021a) Sophoridine inhibits the tumour growth of non-small lung cancer by inducing macrophages m1 polarisation via mapk-mediated inflammatory pathway. Front Oncol 11:634851. https://doi.org/10.3389/fonc.2021.634851
Zhao J, Li P, Zhu H et al (2021b) 7,8-dihydroxyflavone suppresses proliferation and induces apoptosis of human osteosarcoma cells. Acta Biochim Biophys Sin (shanghai) 53(7):903–911. https://doi.org/10.1093/abbs/gmab060
Zhou Q, Wu X, Wen C et al (2018) Toosendanin induces caspase-dependent apoptosis through the p38 mapk pathway in human gastric cancer cells. Biochem Biophys Res Commun 505(1):261–266. https://doi.org/10.1016/j.bbrc.2018.09.093
Funding
This study was supported by the National Key Research and Development Programme Fund (2021YFD1600903-02) and the Jilin Science and Technology Development Fund (20210204062YY).
Author information
Authors and Affiliations
Contributions
AS wrote the manuscript text, SL drew pictures, LL and BQ provided suggestions, and all authors reviewed the manuscript and participated in discussions to form this article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to publish
All participants agree to the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, A., Liu, L., Li, S. et al. Natural products targeting the MAPK-signaling pathway in cancer: overview. J Cancer Res Clin Oncol 150, 6 (2024). https://doi.org/10.1007/s00432-023-05572-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-023-05572-7