Abstract
Purpose
Epidermal growth factor receptors (EGFRs) are overexpressed in a wide range of tumors and are attractive candidates to target in targeted therapies. This study aimed to introduce a novel radiolabeled compound, 177Lu-cetuximab-PAMAM G4, for the treatment of EGFR-expressing tumors.
Methods
In this study, the cetuximab mAb was bound to PAMAM G4 and labeled with 177Lu via DTPA-CHX chelator. The synthesized nanosystem was confirmed by different analyses such as DLS, FT-IR, TEM, and RT-LC. Cell viability of the radioimmunoconjugate was assessed over the EGFR-expressing cell line of SW480. The biodistribution of 177Lu-Cetuximab-PAMAMG4 was determined in different intervals after injection of the radiolabeled compound in normal and tumoral nude mice via scarification and SPECT images.
Results
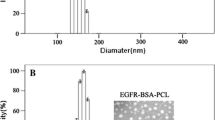
The average size of PAMAM G4 and PAMAM-Cetuximab-DTPA-CHX nanoparticles were 2 and 70 nm, respectively. 177Lu-Cetuximab-PAMAMG4 was prepared with radiochemical purity of more than 98%. The survival rates of SW480 cells at 24, 48, and 72 h post-treatment with177Lu-Cetuximab-PAMAMG4 (500 nM) were 18%, 15%, and 14%, respectively. The biodistribution studies showed a significant accumulation of 177Lu-Cetuximab-PAMAM in the EGFR-expressing tumor.
Conclusion
According to the results, this new agent can be considered as an efficient therapeutic complex for tumors expressing EGFR receptors.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AB:
-
Antibodies
- TGF-α:
-
Alpha-transforming growth factor
- CHO:
-
Chinese hamster ovary cell
- DMSO:
-
Dimethyl sulfoxide
- DLS:
-
Dynamic light scattering
- DTPA:
-
Diethylenetriamine pentaacetate
- EDC:
-
N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride
- EDTA:
-
Ethylenediaminetetraacetic acid
- EGFR:
-
Epidermal growth factor receptor
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- FESEM:
-
Field emission scanning electron microscope
- FTIR:
-
Fourier transform infrared spectroscopy
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPGe:
-
High purity germanium
- ID/g:
-
Injected dose per gram
- mCRC:
-
Metastatic colorectal cancer
- mAb:
-
Monoclonal antibody
- MRI:
-
Magnetic resonance imaging
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide
- NHS:
-
N-hydroxysuccinimide
- NPs:
-
NanoParticles
- PAMAM G4:
-
Polyamidoamine dendrimer generation 4
- PBS:
-
Phosphate buffered saline
- RTLC:
-
Radio thin-layer chromatography
- SPECT:
-
Single photon emission computed tomography
- TEM:
-
Transmission electron microscopy
References
Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B, Dolatabadi JEN, Hamblin MR (2018) PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today 12:177–190. https://doi.org/10.1016/j.apmt.2018.05.002
Alirezapour B, Hadad Dabaghi H, Ansari Ramandi H, Ghamoushi Ramandi A, Hashemizadeh M, Maadi E, Jalilian A, Rajabifar S, Moradkhani S, Masoumi H, Rahiminejad A (2016a) Radiolabeling and quality control of In-111-CHX-A-DTPA-trastuzumab for radioimmunoscintigraphy. Eur J Nucl Med Mol Imaging 43:S461–S461
Alirezapour B, Hadad Dabaghi H, Ghamoushi Ramandi A, Ansari Ramandi H, Soltani N, Hashemizadeh M, Bolorinovin F, Aslan G, Ashtari P (2016b) Radiolabeling and quality control of Ga-67-DTPA-cetuximab for radioimmunoscintigraphy. Eur J Nucl Med Mol Imaging 43:S463–S463
Ayati A, Moghimi S, Salarinejad S, Safavi M, Pouramiri B, Foroumadi A (2020) A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg Chem 99:103811. https://doi.org/10.1016/j.bioorg.2020.103811
Ayyappan S, Prabhakar D, Sharma N (2013) Epidermal growth factor receptor (EGFR)-targeted therapies in esophagogastric cancer. Anticancer Res 33:4139–4155
Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, Jadidi-Niaragh F (2017) Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett 190:64–83. https://doi.org/10.1016/j.imlet.2017.07.015
Bertero A, Boni A, Gemmi M, Gagliardi M, Bifone A, Bardi G (2014) Surface functionalisation regulates polyamidoamine dendrimer toxicity on blood-brain barrier cells and the modulation of key inflammatory receptors on microglia. Nanotoxicology 8:158–168. https://doi.org/10.3109/17435390.2013.765054
Bray F, Laversanne M, Weiderpass E, Soerjomataram I (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127:3029–3030. https://doi.org/10.1002/cncr.33587
Chakravarty R, Chakraborty S, Sarma HD, Nair KVV, Rajeswari A, Das A (2016) 90Y/177Lu-labelled Cetuximab immunoconjugates: radiochemistry optimization to clinical dose formulation. J Label Compd Radiopharm 59:354–363. https://doi.org/10.1002/jlcr.3413
Chen Z, Gao S, Wang D, Song D, Feng Y (2016) Colorectal cancer cells are resistant to anti-EGFR monoclonal antibody through adapted autophagy. Am J Transl Res 8:1190–1196
Cieszykowska I, Zóltowska M, Mielcarski M (2014) Separation of Ytterbium from 177 Lu/Yb mixture by electrolytic reduction and amalgamation. SOP Trans Appl Chem 1:6–13
Dutta T, Jain NK (2007) Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta 1770(4):681–686. https://doi.org/10.1016/j.bbagen.2006.12.007
Dvorakova Z, Henkelmann R, Lin X, Türler A, Gerstenberg H (2008) Production of 177Lu at the new research reactor FRM-II: irradiation yield of 176Lu(n, gamma)177Lu. Appl Radiat Isot 66:147–151
Ferguson KM (2004) Active and inactive conformations of the epidermal growth factor receptor. Biochem Soc Trans 32:742–745. https://doi.org/10.1042/BST0320742
Ferguson KM (2008) Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys 37:353–373. https://doi.org/10.1146/annurev.biophys.37.032807.125829
Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA (2003) EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell 11:507–517. https://doi.org/10.1016/s1097-2765(03)00047-9
Florendo M, Figacz A, Srinageshwar B, Sharma A, Swanson D, Dunbar GL, Rossignol J (2018) Use of polyamidoamine dendrimers in brain diseases. Molecules 23:2238. https://doi.org/10.3390/molecules23092238
Fotouhi P, Sohrabi S, Nosrati N, ZamanVaziri A, Khaleghi S, Narmani A, Jafari H, Mohammadnejad J (2021) Surface modified and rituximab functionalized PAMAM G4 nanoparticle for targeted imatinib delivery to leukemia cells: In vitro studies. Process Biochem 111:221–229
Frechet JM, Tomalia DA (2001) Dendrimers and other dendritic polymers. Wiley, New York
Freidel C (2017) Design, synthesis and application of novel disulfide-intercalation agents for the site-selective modification of peptides and proteins. Johannes Gutenberg-Universität Mainz, Mainz
García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P, Stintzing S (2019) Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front Oncol 9:849. https://doi.org/10.3389/fonc.2019.00849
Gérard HC, Mishra MK, Mao G, Wang S, Hali M, Whittum-Hudson JA, Kannan RM, Hudson AP (2013) Dendrimer-enabled DNA delivery and transformation of Chlamydia pneumoniae. Nanomedicine 9(7):996–1008. https://doi.org/10.1016/j.nano.2013.04.004
Heinz H, Pramanik C, Heinz O, Ding Y, Mishra RK, Marchon D, Flatt RJ, Estrela-Lopis I, Llop J, Moya S, Ziolo RF (2017) Nanoparticle decoration with surfactants: molecular interactions, assembly, and applications. Surf Sci Rep 72:1–58
Huo M, Zhao Y, Liu X, Gao Y, Zhang D, Chang M, Liu M, Xu N, Zhu H (2020) EGFR targeting enhances the efficiency of chemotherapy through inhibiting IRE1α-XBP1s pathway in colorectal cancer cells. J Cancer 11:4464–4473. https://doi.org/10.7150/jca.44234
Ingargiola M, Dittfeld C, Runge R, Zenker M, Heldt JM, Steinbach J, Cordes N, Baumann M, Kotzerke J, Kunz-Schughart LA (2012) Flow cytometric cell-based assay to preselect antibody constructs for radionuclide conjugation. Cytom A 81:865–873. https://doi.org/10.1002/cyto.a.22110
Jain K, Kesharwani P, Gupta U, Jain NK (2010) Dendrimer toxicity: let’s meet the challenge. Int J Pharm 394:122–142. https://doi.org/10.1016/j.ijpharm.2010.04.027
Jeon J (2019) Review of therapeutic applications of radiolabeled functional nanomaterials. Int J Mol Sci 20:2323. https://doi.org/10.3390/ijms20092323
Kandi MR, Mohammadnejad J, Shafiee Ardestani M, Zabihollahi R, Soleymani S, Aghasadeghi MR, Baesi K (2019) Inherent anti-HIV activity of biocompatible anionic citrate-PEG-citrate dendrimer. Mol Biol Rep 46:143–149. https://doi.org/10.1007/s11033-018-4455-6
Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P (2009) IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 37(Database issue):D1006–D1012. https://doi.org/10.1093/nar/gkn838
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7:301–311. https://doi.org/10.1016/j.ccr.2005.03.003
Li JL, Lin SH, Chen HQ, Liang LS, Mo XW, Lai H, Zhang J, Xu J, Gao BQ, Feng Y, Lin Y (2019) Clinical significance of HER2 and EGFR expression in colorectal cancer patients with ovarian metastasis. BMC Clin Pathol 19:3. https://doi.org/10.1186/s12907-019-0085-8
Liao WS, Ho Y, Lin YW, Naveen Raj E, Liu KK, Chen C, Zhou XZ, Lu KP, Chao JI (2019) Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater 86:395–405. https://doi.org/10.1016/j.actbio.2019.01.025
Liko F, Hindré F, Fernandez-Megia E (2016) Dendrimers as innovative radiopharmaceuticals in cancer radionanotherapy. Biomacromol 17:3103–3114
Liu Z, Ma T, Liu H, Jin Z, Sun X, Zhao H, Shi J, Jia B, Li F, Wang F (2014) 177Lu-labeled antibodies for EGFR-targeted SPECT/CT imaging and radioimmunotherapy in a preclinical head and neck carcinoma model. Mol Pharm 11:800–807. https://doi.org/10.1021/mp4005047
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC (2020) Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27:1. https://doi.org/10.1186/s12929-019-0592-z
Lyra ME, Andreou M, Georgantzoglou A, Kordolaimi S, Lagopati N, Ploussi A, Salvara AL, Vamvakas I (2013) Radionuclides used in nuclear medicine therapy—from production to dosimetry. Curr Med Imaging 9:51–75
Makabe K, Yokoyama T, Uehara S, Uchikubo-Kamo T, Shirouzu M, Kimura K, Tsumoto K, Asano R, Tanaka Y, Kumaga I (2021) Anti-EGFR antibody 528 binds to domain III of EGFR at a site shifted from the cetuximab epitope. Sci Rep 11:5790. https://doi.org/10.1038/s41598-021-84171-3
Markatou E, Gionis V, Chryssikos GD, Hatziantoniou S, Georgopoulos A, Demetzos C (2007) Molecular interactions between dimethoxycurcumin and Pamam dendrimer carriers. Int J Pharm 339:231–236. https://doi.org/10.1016/j.ijpharm.2007.02.037
Narmani A, Yavari K, Mohammadnejad J (2017) Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex. Colloids Surf B Biointerfaces 159:232–240. https://doi.org/10.1016/j.colsurfb.2017.07.089
Narmani A, Arani MAA, Mohammadnejad J, Vaziri AZ, Solymani S, Yavari K, Talebi F, Darzi SJ (2020) Breast tumor targeting with PAMAM-PEG-5FU-99mTc as a new therapeutic nanocomplex: in in-vitro and in-vivo studies. Biomed Microdevices 22:31. https://doi.org/10.1007/s10544-020-00485-5
Novy Z, Laznickova A, Mandikova J, Barta P, Laznicek M, Trejtnar F (2014) The effect of chelator type on in vitro receptor binding and stability in 177Lu-labeled cetuximab and panitumumab. J Label Comp Radiopharm 57:448–452. https://doi.org/10.1002/jlcr.3204
Oddone N, Lecot N, Fernández M, Rodriguez-Haralambides A, Cabral P, Cerecetto H, Benech JC (2016) In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J Nanobiotechnol 14:45. https://doi.org/10.1186/s12951-016-0197-6
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S (2002) Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 110:775–787. https://doi.org/10.1016/s0092-8674(02)00963-7
Pearson RM, Sunoqrot S, Hsu HJ, Bae JW, Hong S (2012) Dendritic nanoparticles: the next generation of nanocarriers? Ther Deliv 3:941–959. https://doi.org/10.4155/tde.12.76
Peng X, Kang L, Pang F, Li H, Luo R, Luo X, Sun F (2018) A signal-enhanced lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A. Food Agric Immunol 29:216–227
Sabra R, Billa N, Roberts CJ (2019) Cetuximab-conjugated chitosan-pectinate (modified) composite nanoparticles for targeting colon cancer. Int J Pharm 572:118775. https://doi.org/10.1016/j.ijpharm.2019.118775
Saridaki Z, Georgoulias V, Souglakos J (2010) Mechanisms of resistance to anti-EGFR monoclonal antibody treatment in metastatic colorectal cancer. World J Gastroenterol 16:1177–1187. https://doi.org/10.3748/wjg.v16.i10.1177
Saxena G (2012) Synthesis and characterization of doxorubicin carrying cetuximab-pamam dendrimer bioconjugates
Shanehsazzadeh S, Grüttner C, Yousefnia A, Lahooti A, Gholami A, Nosrati S, Zolghadri S, Anijdan HM, Lotfabadi A, Shiri Varnamkhasti B, Daha FJ, Jalilian A (2016) Development of 177Lu-DTPA-SPIO conjugates for potential use as a dual contrast SPECT/MRI imaging agent. Radiochim Acta 104:337–344
Shigeta K, Hayashida T, Hoshino Y, Okabayashi K, Endo T, Ishii Y, Hasegawa H, Kitagawa Y (2013) Expression of epidermal growth factor receptor detected by cetuximab indicates its efficacy to inhibit in vitro and in vivo proliferation of colorectal cancer cells. PLoS One 8:e66302. https://doi.org/10.1371/journal.pone.0066302
Sickmier EA, Kurzeja RJ, Michelsen K, Vazir M, Yang E, Tasker AS (2016) The Panitumumab EGFR complex reveals a binding mechanism that overcomes cetuximab induced resistance. PLoS One 11:e0163366. https://doi.org/10.1371/journal.pone.0163366
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR in cancer. Mol Oncol 12(1):3–20. https://doi.org/10.1002/1878-0261.12155
Srivastava SC (2002) Bone-seeking therapeutic radiopharmaceuticals. Braz Arch Biol Technol 45:45–55
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Varani M, Galli F, Auletta S, Signore A (2018) Radiolabelled nanoparticles for cancer diagnosis. Clin Transl Imaging 6:271–292. https://doi.org/10.1007/s40336-018-0283-x
Vilalta M, Rafat M, Graves EE (2016) Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci 73:2999–3007. https://doi.org/10.1007/s00018-016-2210-5
Wang L, Qi T, Hu M, Zhang S, Xu P, Qi D, Wu S, Xiao H (2017) Inhibiting mercury re-emission and enhancing magnesia recovery by cobalt-loaded carbon nanotubes in a novel magnesia desulfurization process. Environ Sci Technol 51:11346–11353
Watkins DM, Sayed-Sweet Y, Klimash JW, Turro NJ, Tomalia DA (1997) Dendrimers with hydrophobic cores and the formation of supramolecular dendrimer-surfactant assemblies. Langmuir 13:3136–3141
Xu JM, Wang Y, Wang YL, Wang Y, Liu T, Ni M, Li MS, Lin L, Ge FJ, Gong C, Gu JY, Jia R, Wang HF, Chen YL, Liu RR, Zhao CH, Tan ZL, Jin Y, Zhu YP, Ogino S, Qian ZR (2017) PIK3CA Mutations contribute to acquired cetuximab resistance in patients with metastatic colorectal cancer. Clin Cancer Res 23:4602–4616. https://doi.org/10.1158/1078-0432.CCR-16-2738
Yarden Y (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 37:S3-8. https://doi.org/10.1016/s0959-8049(01)00230-1
Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI (2007) ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Investig 117:2051–2058. https://doi.org/10.1172/JCI32278
Zheng X, Wang T, Jiang H, Li Y, Jiang T, Zhang J, Wang S (2013) Incorporation of Carvedilol into PAMAM-functionalized MWNTs as a sustained drug delivery system for enhanced dissolution and drug-loading capacity. Asian J Pharm Sci 8:278–286
Zhu CN, Chen G, Tian ZQ, Wang W, Zhong W, Li Z, Zhang ZL, Pang D (2017) Near-infrared fluorescent Ag2Se-cetuximab nanoprobes for targeted imaging and therapy of cancer. Small 13:1602309. https://doi.org/10.1002/smll.201602309
Acknowledgements
This study was extracted from an MSc thesis. The authors would like to acknowledge for all the support of NSTRI and the University of Tehran.
Funding
This work was supported by Nuclear Science and Technology Research Institute.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception,design, material preparation, data collection and analysis. The first draft of the manuscript was written by [HY] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The approval of the NSTRI Ethical Committee was obtained for conducting this research.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that all the participants provided informed consent for publication of the images in Figs. 1, 2, 3, 4, 5, 6, 7 and 8.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, S.M., Mohammadnejad, J., Yousefnia, H. et al. Development of 177Lu-Cetuximab-PAMAM dendrimeric nanosystem: a novel theranostic radioimmunoconjugate. J Cancer Res Clin Oncol 149, 7779–7791 (2023). https://doi.org/10.1007/s00432-023-04724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04724-z