Abstract
Early recognition of osteoporosis in children and adolescents is important in order to establish an appropriate diagnosis of the underlying condition and to initiate treatment if necessary. In this review, we present the diagnostic work-up, and its pitfalls, of pediatric patients suspected of osteoporosis including a careful collection of the medical and personal history, a complete physical examination, biochemical data, molecular genetics, and imaging techniques. The most recent and relevant literature has been reviewed to offer a broad overview on the topic. Genetic and acquired pediatric bone disorders are relatively common and cause substantial morbidity. In recent years, there has been significant progress in the understanding of the genetic and molecular mechanistic basis of bone fragility and in the identification of acquired causes of osteoporosis in children. Specifically, drugs that can negatively impact bone health (e.g. steroids) and immobilization related to acute and chronic diseases (e.g. Duchenne muscular dystrophy) represent major risk factors for the development of secondary osteoporosis and therefore an indication to screen for bone mineral density and vertebral fractures. Long-term studies in children chronically treated with steroids have resulted in the development of systematic approaches to diagnose and manage pediatric osteoporosis.
Conclusions: Osteoporosis in children requires consultation with and/or referral to a pediatric bone specialist. This is particularly relevant since children possess the unique ability for spontaneous and medication-assisted recovery, including reshaping of vertebral fractures. As such, pediatricians have an opportunity to improve bone mass accrual and musculoskeletal health in osteoporotic children.
What is Known: • Both genetic and acquired pediatric disorders can compromise bone health and predispose to fractures early in life. • The identification of children at risk of osteoporosis is essential to make a timely diagnosis and start the treatment, if necessary. | |
What is New: • Pediatricians have an opportunity to improve bone mass accrual and musculoskeletal health in osteoporotic children and children at risk of osteoporosis. • We offer an extensive but concise overview about the risk factors for osteoporosis and the diagnostic work-up (and its pitfalls) of pediatric patients suspected of osteoporosis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, osteoporosis in children has been increasingly recognized. Both genetic and acquired pediatric bone disorders can compromise bone strength leading to fractures during childhood. If left untreated, these conditions lead to reduced bone mass, deformities, and impact quality of life, with potential long-term consequences [1, 2]. Awareness among pediatricians is therefore important to identify patients with, or at risk of developing, osteoporosis.
Before the age of 18 years, approximately 95% of the skeletal size and bone and muscle mass is acquired [3]. Therefore, childhood is a very important time to build a strong musculoskeletal system. Factors influencing bone structure and quality are genetic background, organ function, chronic systemic illnesses, medications, and muscular disorders as well as metabolic disorders. Primary osteoporosis usually occurs due to an underlying genetic defect. The most common condition is osteogenesis imperfecta (OI) [4, 5] and more than 24 genes have been identified which cause OI [6]. Secondary, or acquired, osteoporosis develops in children and adults with chronic systemic illnesses due to effects of the disease itself or its treatment. Children and adolescents with osteoporosis can present with a history of recurrent fractures, deformities, or back pain. The accidental finding of vertebral fractures (VFs) on lateral spine radiographs can also lead to the diagnosis of osteoporosis [7, 8].
In both primary and secondary osteoporosis, hidden vertebral fractures delay the diagnosis of osteoporosis. Early identification of VFs through lateral spine imaging should lead to referral to a specialist, because early treatment has the potential to prevent future fractures. In this review, we will discuss normal bone physiology, the definition of osteoporosis and will particularly focus on the diagnostic work-up, and its pitfalls, of children suspected of osteoporosis using clinical signs, biochemistry, molecular genetic testing, and imaging techniques.
Normal bone physiology
Bone is a dynamic tissue, and the growth in length and width (modeling) and the remodeling of bone are complex processes. Here we will discuss the key features.
Bone is composed of minerals (50–70%, mostly hydroxyapatite), organic matrix (20–40%, mostly collagen), water (5–10%), and lipids (< 3%) [9]. The mineral content lends strength and rigidity to the bone, while the organic matrix is responsible for its elasticity and toughness [10, 11]. Most of the skeleton consists of cortical bone (~ 80%), while the inner skeletal compartment is composed of a honeycomb-like structure known as trabecular bone. Although trabecular bone represents only 20% of total bone mass, its surface area is far greater than that of cortical bone and its turnover is more dynamic. Consequently, bone loss in regions that are mainly composed of the more metabolically active trabecular bone (e.g. vertebrae, hip) are more susceptible for true osteoporotic fractures [12].
Bone tissue contains several cell types, namely, osteoclasts (break down bone matrix), osteoblasts (promote formation of new bone tissue), osteocytes (orchestrate the activity of osteoclasts and osteoblasts in response to mechanical strain, and also build bone), osteomorphs (involved in the regulation of bone resorption), and bone lining cells [13,14,15,16]. Bone modelling summarizes all the processes involved in growth and shaping of new bone, including the bone formation needed for bone elongation and widening (growth), metaphyseal inwaisting (the shaping of the end of long bones), and modelling drift of pelvic bone. Bone formation by osteoblasts or osteocytes includes the secretion of osteoid, mostly consisting of type I collagen, and its mineralization to form mature bone matrix. Bone remodeling involves old-by-new replacement in three consecutive phases with osteoclast-mediated resorption of existing bone and the consequent release of calcium and phosphate, the reversal phase in which osteoblast cells appear on the bone surface, and lastly, the osteoblast-mediated synthesis of osteoid that will undergo mineralization to form mature bone matrix [14, 16].
The molecular pathways that regulate bone formation, resorption, and remodeling are complex and their discussion is beyond the scope of this review. We will briefly focus on the RANK (Receptor Activator of Nuclear Factor Kappa B)–RANKL (RANK Ligand) system, WNT-signaling (Wingless iNTegration site family), and TGF-β (Transforming Growth Factor-β) signaling pathway.
One of the principal regulatory pathways is the RANK–RANKL–OPG (osteoprotegerin) system. RANK is expressed on the surface of osteoclast precursors and RANKL is expressed by osteoblasts and osteocytes. When RANKL binds with RANK, cell differentiation of osteoclast precursors is activated and consequently osteoclast-mediated bone resorption [17]. Osteoblasts also express OPG, a decoy receptor that binds to RANKL. By preventing the interaction between RANK and RANKL, RANK activation is inhibited and bone resorption is prevented [18]. Systemic regulators involved in this pathway include, amongst others, parathyroid hormone (PTH), active vitamin D, glucocorticoids, growth hormone, and sex hormones. Also, cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor can activate this bone resorption pathway.
The WNT signaling pathway promotes osteogenesis and increases bone mass by suppressing apoptosis in osteoblast precursor cells and facilitating osteoblast differentiation. The WNT pathway is activated by ligands, such as Wnt1 and Wnt3a, through their binding to the transmembrane Frizzled receptors and LRP (low-density lipoprotein receptor-related protein)-5 and LRP-6 complexes. As OPG opposes RANK, sclerostin (secreted by osteocytes) inhibits the WNT signaling pathway through its binding to LRP-5 and LRP-6 [19].
Osteogenesis is enhanced by the TGF-β signaling pathway that promotes the recruitment, proliferation and differentiations of progenitor cells into osteoblasts. TGF-β is mainly secreted by the extracellular matrix and osteoclasts can increase its secretion to balance bone resorption. Also, the TGF-β pathway interacts with the WNT signaling through the inhibition of sclerostin secretion and the upregulations of several WNT ligands [20, 21].
Definition of osteoporosis
According to the International Society for Clinical Densitometry (ISCD), pediatric osteoporosis is currently defined by (1) the combination of a bone mineral density (BMD) Z-score ≤ −2 and a clinically significant fracture history defined as the presence of either two or more long bone fractures before the age of 10 years or three or more long bone fractures at any age up to 19 years; or (2) one or more vertebral compression fractures occurring without high energy trauma or local disease irrespective of the BMD Z-score [22, 23].
As childhood fractures are very common [24,25,26], this definition aims to distinguish children with an underlying condition from those who experience fractures as a result of typical childhood behavior or non-accidental trauma. There are, however, several challenges in using this definition. For example, the inclusion of a BMD Z-score cut-off of ≤ –2 in defining osteoporosis. Depending on the reference data used to calculate the BMD Z-score, this score can differ by as much as 2 SD [27,28,29]. Another challenge is the risk of underdiagnosing conditions predisposing to osteoporosis, e.g. whilst waiting for the second or third fracture in children with low BMD or because the BMD Z-score is above −2 despite recurrent fractures. Therefore, in line with current recommendations, diagnosing osteoporosis should not be based on BMD alone but take into account the clinical context, specifically the severity and prognosis of the underlying disease or treatment [30].
Primary osteoporosis
Primary osteoporosis refers to conditions of heritable bone fragility caused by intrinsic skeletal defects with abnormal composition of bone tissue. Causative genes affect different pathways such as collagen type I synthesis, bone mineralization, osteoblasts, or osteocyte dysfunction [31, 32]. Children with primary osteoporosis comprise a heterogeneous group with a broad spectrum of skeletal and extraskeletal characteristics, ranging from mild to lethal forms. These conditions result in severe bone disease and low bone mass accrual. Timely recognition is therefore important to initiate treatment and specialist care [33,34,35].
OI is the most common form of primary osteoporosis. The main clinical features are recurrent fractures, skeletal deformities, short stature, blue sclera, dentinogenesis imperfecta, hearing loss, and ligamentous laxity; however, these can vary among patients depending on the type of OI [36]. Inheritance of the most frequent types of OI is autosomal dominant (type 1–5; 85–90% are caused by COL1A1, COL1A2, or IFITM5 mutations), while rarer forms show autosomal recessive or X-linked inheritance [4, 36,37,38]. More rare primary osteoporotic conditions are described in Table 1 [33, 39,40,41,42,43,44,45].
In contrast, Idiopathic Juvenile Osteoporosis (IJO) is a condition with unknown pathophysiology. IJO is characterized by pain in the back, hips and/or lower limbs and difficulty walking, as well as vertebral compression fractures and long bone fractures. The onset of symptoms is insidious and usually starts before puberty. Interestingly, during puberty the symptoms may improve [46, 47]. In contrast to most genetic causes of osteoporosis, there is no positive family history, no extraskeletal manifestations and no growth impairment. With further molecular genetic advancements, the diagnosis of IJO is expected to diminish. Already, heterozygous mutations of LRP5 have been described in some cases [48]. To date, the diagnosis of IJO remains a clinical one and based on exclusions of other causes of osteoporosis [46, 47].
Secondary osteoporosis
Secondary osteoporosis occurs as a result of systemic underlying conditions or medications. The most common causes include inflammatory disorders, hematological and oncological disorders, renal disease, immobility or muscle impairment and medications such as corticosteroids (Table 2). At any age, malnutrition, immobilization, and lack of physical activity represent additional risk factors for osteoporosis development [7, 49,50,51,52,53,54,55,56,57].
Depending on the underlying cause, the pathophysiology of osteoporosis differs. For example, 16% of children with acute lymphoblastic leukemia already have VFs at diagnosis, likely caused by the release of cytokines from leukemic cells that stimulate osteoclast activity [8]. The risk of fractures is the highest in the first two years of diagnosis and the presence of VFs at diagnosis is highly predictive of future fractures. Up to 45% of children presenting with VFs at the diagnosis can be asymptomatic; therefore, lateral spine imaging is advised to detect asymptomatic VFs, predict the risk of future fractures, and prevent vertebral deformities and long-term morbidity [58,59,60].
Immobility is another frequent cause of osteoporosis. According to the mechanostat theory, bone strength is regulated by muscle force. During immobilization, lack of muscle tension results in reduced biomechanical bone loading, which is sensed by osteocytes and translated into biochemical signals that lead to thinner long bones and less trabecular bone formation [7, 13, 61]. Hence, children with for example cerebral palsy have reduced periosteal apposition in lower extremity bones, resulting in reduced cortical thickness. Consequently, fractures occur most commonly in the distal femur and tibia [62,63,64]. Also, children affected by Duchenne Muscular Dystrophy require a careful follow-up by a pediatric bone specialist because of immobility, long-term steroid treatment, and hypogonadism [63, 65].
The detrimental effect of glucocorticosteroids (GCs) on bone is caused by an initial phase of increased bone resorption followed by a phase of decreased bone formation [66,67,68,69]. In children, high cumulative doses of intravenous and/or oral corticosteroids and repeated pulse therapy have been associated with the development of osteoporosis [70]. There are no conclusive data available on the effect of low and medium doses of steroids; however, from data acquired in the adult population, the chronic administration of systemic corticosteroids at a medium to low dose is also suspected to impair growth and affect bone formation [71, 72]. Furthermore, long-term inhaled corticosteroid (ICS) therapy in children may negatively affect BMD. This was seen in children treated with high doses of ICS but not in children treated with low and medium doses [73,74,75]. However, fracture rate is not increased in children on ICS when adjusted for asthma severity [76].
With improving survival rates in many systemic conditions, complications such as osteoporosis are on the rise, and hence, monitoring of bone health should be part of the standardized follow-up [77]. In some subjects presenting with low impact fractures or back pain, however, the underlying disease is not yet known and they present with signs of impaired bone health.
Clinical signs and laboratory work-up
In a child suspected of or with osteoporosis, a thorough medical history needs to be taken. History of fractures (number, localization, mechanism, and radiographic features) must be investigated. Back pain needs to be questioned because it might be a sign of vertebral fractures. Furthermore, a detailed history of comorbidities, physical activity, diet and medications, growth and puberty, and family history (e.g. fractures, hearing loss) needs to be taken. Physical examination should include anthropometry including head circumference, body proportions, assessment of teeth, sclera, joint laxity, scoliosis, limb deformities, widening of the wrists and ankles, spine tenderness, skin laxity, and pubertal status.
In every child referred for assessment of bone health-selected laboratory studies of bone mineralization should be performed including serum calcium, phosphate, magnesium, creatinine, alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), 25-hydroxy vitamin D, PTH, and urinary creatinine, calcium, and phosphate. Whilst this biochemical assessment excludes bone hypomineralization disorders (all types of rickets/osteomalacia), there is no current blood test that can diagnose or exclude osteoporosis apart from molecular genetic testing. Where no secondary cause of osteoporosis can be found, targeted-, whole exome-, and RNA sequencing methods should be considered to search for a genetic cause [78].
In rare cases, fractures or bone pain may be the first presenting symptom of an underlying condition. Therefore, erythrocyte sedimentation rate, a full blood count with leucocyte differentiation, serum TSH, free T4, and celiac screening is also advised. If hypogonadism is suspected, the LH, FSH, testosterone (♂), or estradiol (♀) should be checked; if Cushing’s disease is suspected, 24-h urinary cortisol should be checked. This work-up should be tailored to the presenting symptoms and population-appropriate pediatric reference data used [7, 39].
Diagnostic techniques to assess bone health
The main imaging techniques used to assess bone health in children are dual-energy X-ray absorptiometry (DXA) and conventional lateral spine radiographs. The other techniques described below are mainly reserved for research.
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) is the most commonly used technique for assessing bone mass in children due to its worldwide availability, precision, reproducibility, and availability of normative data [23, 79]. In children, the preferred measurement sites are the lumbar spine (LS) and the total body less head (TBLH) [23, 80]. DXA-derived values for children are expressed as age-specific and sex-specific Z-scores. Normative pediatric data must be used for Z-score calculation, which are available for children older than 3 years for the TBLH, while LS measurements are feasible and reproducible also for children aged < 3 years [81].
Pitfalls in DXA measurement are common as it is a 2-dimensional technique. For example, DXA measurements underestimate BMD (g/cm2) in children with short stature or pubertal delay and appropriate methods to account for growth delay when interpreting DXA results for children < 5 years are currently unknown. Hence, adjustment for bone size or skeletal size is mandatory. To do so, volumetric BMD (vBMD or bone mineral apparent density [BMAD], g/cm3) is calculated or BMD Z-scores are adjusted for height [27, 82]. In addition, disrupting factors, such as movement during measurement, scoliosis, and metalwork, can give non-interpretable results [83]. If DXA-LS cannot be performed, alternative sites are the distal forearm, the proximal hip, and the lateral distal femur [30]. Despite these pitfalls, DXA remains the technique of choice to measure bone mass.
Radiogrammetry
To assess bone health on digital radiographs, different parameters have been proposed such as the Bone Health Index® (BHI) [84, 85]. Studies comparing BHI determined by X-ray and BMD assessed by DXA showed contradictory results [86,87,88,89,90,91,92]. BHI seems to overestimate bone health impairment, also, the correlation with DXA measurements is not always good as it applies mainly to absolute values than to Z-scores. Therefore, its use is not currently recommended.
Radiography
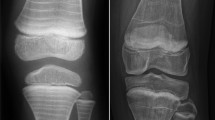
Radiography is used to detect VFs and scoliosis. In contrast to adult guidelines, where back pain alone does not represent an indication to perform imaging, lateral spine imaging (thoracic and lumbar vertebrae) should be performed in all children with suspected osteoporosis and hidden VFs should be investigated [93, 94]. VFs are usually assessed through the Genant’s semi-quantitative method. This method is based on the estimation of the vertebral height loss and the visual evaluation of morphological change. A vertebral height loss > 20% indicates a VF, with 20–25% height loss defined as mild, 26–40% as moderate, and > 40% as severe (Fig. 1) [95,96,97]. Recent studies have shown that the newest generation of DXA scanners can also detect moderate to severe VFs in children through the vertebral fracture assessment (VFA), using a lower doses of radiation than lateral spine radiographs (Fig. 2) [98,99,100].
a Male adolescent with backpain, no history of trauma reported. Lateral radiograph of the spine shows a vertebral fracture of the 12th thoracic vertebra. There is a 35% loss of height, in keeping with a grade 2 fracture according to the Genant classification (moderate fracture, 25 to 40% loss of height). The 11th thoracic vertebra and the 1st lumbar vertebra also show mild wedging; measurements are not shown to prevent clutter of the image. b Girl with juvenile osteoporosis. Lateral radiograph of the spine shows multiple fractures. Measurement shows a 34% loss of height, in keeping with a grade 2 fracture according to the Genant classification (moderate fracture, 25 to 40% loss of height)
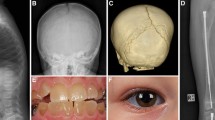
Infant with Osteoporosis-pseudoglioma syndrome (LRP5 mutation) treated with bisphosphonates. a Lateral spine radiograph shows multiple vertebral fractures of the thoracic and lumbar spine. There are dense vertebral endplates as a result of bisphosphonate treatment. b Although DXA of the lumbar spine shows a low BMD, it is underestimating the severity of the disease due to the loss of height and the increased density of the vertebral endplates. c Automated DXA vertebral fracture assessment (VFA)
Quantitative computed tomography
Quantitative computed tomography (QCT), peripheral QCT (pQCT), and vertebral QCT (vQCT) are able to assess cortical and trabecular bone separately, vBMD rather than aBMD and provide information on bone geometry, impossible to obtain with DXA [62].
pQCT devices evaluate bone at the level of radius or tibia [101] and can be used as an alternative in children with severe scoliosis that cannot undergo DXA-LS. Main limitations are related to the need of proper positioning of the patient to achieve reproducibility and movements during the scan can result in artefacts. Furthermore, whether pQCT measurements adequately reflect the whole skeleton, including the spine, is under debate. Reference data are available [102,103,104] but have their limitations [105].
Other diagnostic techniques
Magnetic resonance imaging (MRI) is not routinely used in clinical practice yet, but presents several advantages. MRI provides volumetric bone measures and can separately evaluate cortical and trabecular bone like QCT. In addition to QCT, MRI can scan both axial and peripheral skeleton at the same time and acquire data from multiple anatomical planes with no need to reposition the patient. However, the time of acquisition of images is long (around 20–30 min), the closed space could cause stress, sedation is required for younger children, and the costs are high [106].
Quantitative ultrasonography (QUS) has been proposed as a useful tool to assess bone mineral status from early childhood to young-adulthood with a very small confounding effect related to bone size [107, 108]. Nevertheless, its use is not recommended in pediatric population with the exception of research settings [109, 110].
In children with a history of frequent low impact fractures and unclear causation, a trans-iliac bone biopsy with tetracycline double-labeling may be indicated. Structural and dynamic parameters of bone quality can be obtained through histomorphometry and tissue density assessed using backscattered electron microscopy. Bone biopsy, however, is infrequently performed because it is invasive, requires anesthesia, and is performed only in specialized centers [7, 49, 111].
Conclusion
Increased awareness among pediatricians is important as both genetic and acquired pediatric bone disorders cause substantial morbidity and require early detection. Osteoporosis in children requires consultation with and/or referral to a pediatric bone specialist. This is particularly relevant since children possess the unique ability for spontaneous and medication-assisted recovery, including reshaping of vertebral fractures. As such, pediatricians have an opportunity to improve bone mass accrual and musculoskeletal health in osteoporotic children.
OI represents the main cause of primary osteoporosis, but many more rare genetic conditions affecting bone health are recognized that require specialist management. In children at risk of primary and secondary osteoporosis, it is mandatory to check for vertebral fractures. At first presentation, a careful history and examination should be taken and bone hypomineralization disorders excluded by laboratory investigations. To date, DXA and conventional X-radiographs are the techniques of choice to assess bone health and diagnose vertebral fractures. We emphasize that DXA interpretation requires pediatric expertise and discourage DXA use in children outside specialist centers. The flowchart in Fig. 3 summarizes the main steps that the pediatricians should take when evaluating a child suspected of bone fragility.
Availability of data and material
All articles included in the review are cited among the references.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BHI:
-
Bone health index
- BMAD:
-
Bone mineral apparent density
- BMD:
-
Bone mineral density
- DXA:
-
Dual-energy x-ray absorptiometry
- VFs:
-
Vertebral fractures
- GGT:
-
Gamma glutamyl transferase
- GCs:
-
Glucocorticosteroids
- ICS:
-
Inhaled corticosteroid
- IJO::
-
Idiopathic juvenile osteoporosis
- IL:
-
Interleukine
- LRP:
-
Low-density lipoprotein receptor-related protein
- LS:
-
Lumbar spine
- MRI:
-
Magnetic resonance imaging
- OI:
-
Osteogenesis imperfect
- OPG:
-
Osteoprotegerin
- pQCT:
-
Peripheral QCT
- PTH:
-
Parathyroid hormone
- QCT:
-
Quantitative computed tomography
- QUS:
-
Quantitative ultrasonography
- RANK:
-
Receptor activator of nuclear factor kappa B
- RANKL:
-
RANK ligand
- TBLH:
-
Total (whole) body less head
- TGF-β:
-
Transforming growth factor-β
- vBMD:
-
Volumetric BMD
- vQCT:
-
Vertebral QCT
- WNT:
-
Wingless iNTegration site family
References
Boyce AM, Gafni RI (2011) Approach to the child with fractures. J Clin Endocrinol Metab 96:1943–1952. https://doi.org/10.1210/jc.2010-2546
Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK (2017) The determinants of peak bone mass. J Pediatr 180:261–269. https://doi.org/10.1016/j.jpeds.2016.09.056
Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26:1729–1739. https://doi.org/10.1002/jbmr.412
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557. https://doi.org/10.1038/nrendo.2011.81
Claeys L, Storoni S, Eekhoff M, Elting M, Wisse L, Pals G, Bravenboer N, Maugeri A, Micha D (2021) Collagen transport and related pathways in osteogenesis imperfecta. Hum Genet 140:1121–1141. https://doi.org/10.1007/s00439-021-02302-2
Van Dijk FS, Pals G, Van Rijn RR, Nikkels PG, Cobben JM (2010) Classification of osteogenesis imperfecta revisited. Eur J Med Genet 53:1–5. https://doi.org/10.1016/j.ejmg.2009.10.007
Saraff V, Hogler W (2015) Osteoporosis in children: Diagnosis and management. Eur J Endocrinol 173:R185-197. https://doi.org/10.1530/EJE-14-0865
Huber AM, Gaboury I, Cabral DA, Lang B, Ni A, Stephure D, Taback S, Dent P, Ellsworth J, LeBlanc C, Saint-Cyr C, Scuccimarri R, Hay J, Lentle B, Matzinger M, Shenouda N, Moher D, Rauch F, Siminoski K, Ward LM (2010) Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res 62:516–526. https://doi.org/10.1002/acr.20171
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(Suppl 3):S131-139. https://doi.org/10.2215/CJN.04151206
Landis WJ (1995) The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16:533–544. https://doi.org/10.1016/8756-3282(95)00076-p
Tzaphlidou M, Berillis P (2005) Collagen fibril diameter in relation to bone site. A quantitative ultrastructural study. Micron 36:703–705. https://doi.org/10.1016/j.micron.2005.05.012
Ott SM (2018) Cortical or trabecular bone: what’s the difference? Am J Nephrol 47:373–375. https://doi.org/10.1159/000489672
El-Gazzar A, Hogler W (2021) Mechanisms of bone fragility: from osteogenesis imperfecta to secondary osteoporosis. Int J Mol Sci. https://doi.org/10.3390/ijms22020625
Ganesan K, Jandu JS, Roane D (2021) Secondary Osteoporosis. StatPearls
McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, Kyaw W et al (2021) Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184(1330–1347):e1313. https://doi.org/10.1016/j.cell.2021.02.002
Wang K, Ren Y, Lin S, Jing Y, Ma C, Wang J, Yuan XB, Han X, Zhao H, Wang Z, Zheng M, Xiao Y, Chen L, Olsen BR, Feng JQ (2021) Osteocytes but not osteoblasts directly build mineralized bone structures. Int J Biol Sci 17:2430–2448. https://doi.org/10.7150/ijbs.61012
Nagy V, Penninger JM (2015) The RANKL-RANK story. Gerontology 61:534–542. https://doi.org/10.1159/000371845
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319. https://doi.org/10.1016/s0092-8674(00)80209-3
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayama T, Saito M, Marumo K (2019) The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. https://doi.org/10.3390/ijms20225525
Amjadi-Moheb F, Akhavan-Niaki H (2019) Wnt signaling pathway in osteoporosis: Epigenetic regulation, interaction with other signaling pathways, and therapeutic promises. J Cell Physiol. https://doi.org/10.1002/jcp.28207
Halloran D, Durbano HW, Nohe A (2020) Bone morphogenetic protein-2 in development and bone homeostasis. J Dev Biol. https://doi.org/10.3390/jdb8030019
Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O, Munns CF, Shaw N, International Society of Clinical D (2014) Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom 17:275–280. https://doi.org/10.1016/j.jocd.2014.01.004
Gordon CM, Leonard MB, Zemel BS (2014) 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 17:219–224. https://doi.org/10.1016/j.jocd.2014.01.007
Larsen AV, Mundbjerg E, Lauritsen JM, Faergemann C (2020) Development of the annual incidence rate of fracture in children 1980–2018: a population-based study of 32,375 fractures. Acta Orthop 91:593–597. https://doi.org/10.1080/17453674.2020.1772555
Berthold O, Frericks B, John T, Clemens V, Fegert JM, Moers AV (2018) Abuse as a cause of childhood fractures. Dtsch Arztebl Int 115:769–775. https://doi.org/10.3238/arztebl.2018.0769
Landin LA (1997) Epidemiology of children’s fractures. J Pediatr Orthop 6:79–83. https://doi.org/10.1097/01202412-199704000-00002
Kocks J, Ward K, Mughal Z, Moncayo R, Adams J, Hogler W (2010) Z-score comparability of bone mineral density reference databases for children. J Clin Endocrinol Metab 95:4652–4659. https://doi.org/10.1210/jc.2010-0677
Ma J, Siminoski K, Alos N, Halton J, Ho J, Lentle B, Matzinger M, Shenouda N, Atkinson S, Barr R, Cabral DA, Couch R, Cummings EA, Fernandez CV, Grant RM, Rodd C, Sbrocchi AM, Scharke M, Rauch F, Ward LM, Canadian SC (2015) The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J Clin Endocrinol Metab 100:1018–1027. https://doi.org/10.1210/jc.2014-3096
Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI (1999) Discrepancies in pediatric bone mineral densityreference data: Potential for misdiagnosis ofosteopenia. J Pediatr 135:182–188. https://doi.org/10.1016/s0022-3476(99)70020-x
Ward LM, Weber DR, Munns CF, Hogler W, Zemel BS (2020) A contemporary view of the definition and diagnosis of osteoporosis in children and adolescents. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz294
Arundel P, Bishop N (2015) Primary osteoporosis. Endocr Dev 28:162–175. https://doi.org/10.1159/000381037
Boudin E, Fijalkowski I, Hendrickx G, Van Hul W (2016) Genetic control of bone mass. Mol Cell Endocrinol 432:3–13. https://doi.org/10.1016/j.mce.2015.12.021
Marrani E, Giani T, Simonini G, Cimaz R (2017) Pediatric osteoporosis: Diagnosis and treatment considerations. Drugs 77:679–695. https://doi.org/10.1007/s40265-017-0715-3
Kampe AJ, Makitie RE, Makitie O (2015) New genetic forms of childhood-onset primary osteoporosis. Horm Res Paediatr 84:361–369. https://doi.org/10.1159/000439566
Makitie O, Zillikens MC (2021) Early-onset osteoporosis. Calcif Tissue Int. https://doi.org/10.1007/s00223-021-00885-6
van Dijk FS (2015) Genetics of osteoporosis in children. Endocr Dev 28:196–209. https://doi.org/10.1159/000381046
Bregou Bourgeois A, Aubry-Rozier B, Bonafe L, Laurent-Applegate L, Pioletti DP, Zambelli PY (2016) Osteogenesis imperfecta: from diagnosis and multidisciplinary treatment to future perspectives. Swiss Med Wkly 146:w14322. https://doi.org/10.4414/smw.2016.14322
Tournis S, Dede AD (2018) Osteogenesis imperfecta - a clinical update. Metabolism 80:27–37. https://doi.org/10.1016/j.metabol.2017.06.001
Ward LM, Konji VN, Ma J (2016) The management of osteoporosis in children. Osteoporos Int 27:2147–2179. https://doi.org/10.1007/s00198-016-3515-9
Rauch F, Fahiminiya S, Majewski J, Carrot-Zhang J, Boudko S, Glorieux F, Mort JS, Bachinger HP, Moffatt P (2015) Cole-Carpenter syndrome is caused by a heterozygous missense mutation in P4HB. Am J Hum Genet 96:425–431. https://doi.org/10.1016/j.ajhg.2014.12.027
Yasukawa S, Uehara M, Suzuki T, Nakano M, Kosho T, Nakamura Y, Takahashi J (2021) The first experience of denosumab therapy on patients with Ehlers-Danlos syndrome and osteoporosis: Detailed observation of two patients. Mod Rheumatol Case Rep 5:377–383. https://doi.org/10.1080/24725625.2020.1799493
Grover M, Brunetti-Pierri N, Belmont J, Phan K, Tran A, Shypailo RJ, Ellis KJ, Lee BH (2012) Assessment of bone mineral status in children with Marfan syndrome. Am J Med Genet A 158A:2221–2224. https://doi.org/10.1002/ajmg.a.35540
Papadopoulos I, Bountouvi E, Attilakos A, Gole E, Dinopoulos A, Peppa M, Nikolaidou P, Papadopoulou A (2019) Osteoporosis-pseudoglioma syndrome: Clinical, genetic, and treatment-response study of 10 new cases in Greece. Eur J Pediatr 178:323–329. https://doi.org/10.1007/s00431-018-3299-3
Rudolph G, Kalpadakis P, Bettecken T, Lichtner P, Haritoglou C, Hergersberg M, Meitinger T, Schmidt H (2003) Spondylo-ocular syndrome: a new entity with crystalline lens malformation, cataract, retinal detachment, osteoporosis, and platyspondyly. Am J Ophthalmol 135:681–687. https://doi.org/10.1016/s0002-9394(02)02155-4
Weber DR, Coughlin C, Brodsky JL, Lindstrom K, Ficicioglu C, Kaplan P, Freehauf CL, Levine MA (2016) Low bone mineral density is a common finding in patients with homocystinuria. Mol Genet Metab 117:351–354. https://doi.org/10.1016/j.ymgme.2015.12.003
Bacchetta J, Wesseling-Perry K, Gilsanz V, Gales B, Pereira RC, Salusky IB (2013) Idiopathic juvenile osteoporosis: a cross-sectional single-centre experience with bone histomorphometry and quantitative computed tomography. Pediatr Rheumatol 11:1–8. https://doi.org/10.1186/1546-0096-11-6
Franceschi R, Vincenzi M, Camilot M, Antoniazzi F, Freemont AJ, Adams JE, Laine C, Makitie O, Mughal MZ (2015) Idiopathic juvenile osteoporosis: Clinical experience from a single centre and screening of LRP5 and LRP6 genes. Calcif Tissue Int 96:575–579. https://doi.org/10.1007/s00223-015-9983-7
Hartikka H, Makitie O, Mannikko M, Doria AS, Daneman A, Cole WG, Ala-Kokko L, Sochett EB (2005) Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res 20:783–789. https://doi.org/10.1359/JBMR.050101
Sakka SD, Cheung MS (2020) Management of primary and secondary osteoporosis in children. Ther Adv Musculoskelet Dis 12:1759720X20969262. https://doi.org/10.1177/1759720X20969262
Grover M, Bachrach LK (2017) Osteoporosis in children with chronic illnesses: Diagnosis, monitoring, and treatment. Curr Osteoporos Rep 15:271–282. https://doi.org/10.1007/s11914-017-0371-2
Sahin S, Sadri S, Baslar Z, Ar MC (2019) Osteoporosis in patients with hemophilia: Single-center results from a middle-income country. Clin Appl Thromb Hemost 25:1076029619861689. https://doi.org/10.1177/1076029619861689
Schipper LG, Fleuren HW, van den Bergh JP, Meinardi JR, Veldman BA, Kramers C (2015) Treatment of osteoporosis in renal insufficiency. Clin Rheumatol 34:1341–1345. https://doi.org/10.1007/s10067-015-2883-4
Gulati S, Godbole M, Singh U, Gulati K, Srivastava A (2003) Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease? Am J Kidney Dis 41:1163–1169. https://doi.org/10.1016/s0272-6386(03)00348-2
Augoulea A, Zachou G, Lambrinoudaki I (2019) Turner syndrome and osteoporosis. Maturitas 130:41–49. https://doi.org/10.1016/j.maturitas.2019.09.010
Ferlin A, Schipilliti M, Di Mambro A, Vinanzi C, Foresta C (2010) Osteoporosis in Klinefelter’s syndrome. Mol Hum Reprod 16:402–410. https://doi.org/10.1093/molehr/gaq026
Tamminen IS, Valta H, Jalanko H, Salminen S, Mayranpaa MK, Isaksson H, Kroger H, Makitie O (2014) Pediatric solid organ transplantation and osteoporosis: a descriptive study on bone histomorphometric findings. Pediatr Nephrol 29:1431–1440. https://doi.org/10.1007/s00467-014-2771-1
Galindo-Zavala R, Bou-Torrent R, Magallares-Lopez B, Mir-Perello C, Palmou-Fontana N, Sevilla-Perez B, Ildefonso MS, Gonzalez-Fernandez MI, Roman-Pascual A, Alcaniz-Rodriguez P, Nieto-Gonzalez JC, Lopez-Corbeto M, Grana-Gil J (2020) Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J 18:20. https://doi.org/10.1186/s12969-020-0411-9
Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, Shenouda N et al (2018) Bone morbidity and recovery in children with acute lymphoblastic leukemia: Results of a six-year prospective cohort study. J Bone Miner Res 33:1435–1443. https://doi.org/10.1002/jbmr.3447
Mostoufi-Moab S, Ward LM (2019) Skeletal morbidity in children and adolescents during and following cancer therapy. Horm Res Paediatr 91:137–151. https://doi.org/10.1159/000494809
Verwaaijen EJ, Ma J, de Groot-Kruseman HA, Pieters R, van der Sluis IM, van Atteveld JE, Halton J et al (2021) A validated risk prediction model for bone fragility in children with acute lymphoblastic leukemia. J Bone Miner Res. https://doi.org/10.1002/jbmr.4442
Zacharin M (2009) Assessing the skeleton in children and adolescents with disabilities: avoiding pitfalls, maximising outcomes. A guide for the general paediatrician. J Paediatr Child Health 45:326–331. https://doi.org/10.1111/j.1440-1754.2009.01506.x
Binkley T, Johnson J, Vogel L, Kecskemethy H, Henderson R, Specker B (2005) Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr 147:791–796. https://doi.org/10.1016/j.jpeds.2005.07.014
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR (2018) Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics 142:S34–S42. https://doi.org/10.1542/peds.2018-0333E
Crabtree NJ, Adams JE, Padidela R, Shaw NJ, Hogler W, Roper H, Hughes I, Daniel A, Mughal MZ (2018) Growth, bone health & ambulatory status of boys with DMD treated with daily vs. intermittent oral glucocorticoid regimen. Bone 116:181–186. https://doi.org/10.1016/j.bone.2018.07.019
Weber DR, Thomas S, Erickson SW, Fox D, Oleszek J, Pandya S, Venkatesh Y, Westfield C, Ciafaloni E, Muscular Dystrophy T, Research N (2018) Bone Health and endocrine care of boys with duchenne muscular dystrophy: data from the MD STARnet. J Neuromuscular Dis 5:497–507. https://doi.org/10.3233/JND-180317
von Scheven E, Corbin KJ, Stagi S, Cimaz R (2014) Glucocorticoid-associated osteoporosis in chronic inflammatory diseases: Epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep 12:289–299. https://doi.org/10.1007/s11914-014-0228-x
Weinstein RS (2011) Glucocorticoid-induced bone disease. N Engl J Med 365:62–70. https://doi.org/10.1056/NEJMcp1012926
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos Int 18:1319–1328. https://doi.org/10.1007/s00198-007-0394-0
Engvall IL, Svensson B, Tengstrand B, Brismar K, Hafstrom I (2008) Impact of low-dose prednisolone on bone synthesis and resorption in early rheumatoid arthritis: Experiences from a two-year randomized study. Arthritis Res Ther 10:R128. https://doi.org/10.1186/ar2542
Tantawy AA, El Bostany EA, Matter RM, El Ghoroury EA, Ragab S, El Sherif NH (2013) Bone mass and biochemical markers of bone turnover in children and adolescents with chronic immune thrombocytopenia: Relation to corticosteroid therapy and vitamin D receptor gene polymorphisms. Platelets 24:282–287. https://doi.org/10.3109/09537104.2012.690544
Van Staa TP, Leufkens HGM, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787. https://doi.org/10.1007/s001980200108
De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP (2007) Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 56:208–214. https://doi.org/10.1002/art.22294
Anuradha KWDA, Prematilake GLDC, Batuwita BAUI, Kannangoda KASR, Hewagamage US, Wijeratne S, Lankatilake K, De Silva KSH (2019) Effect of long term inhaled corticosteroid therapy on adrenal suppression, growth and bone health in children with asthma. BMC Pediatr 19:411. https://doi.org/10.1186/s12887-019-1760-8
Skoner DP (2016) Inhaled corticosteroids: Effects on growth and bone health. Ann Allergy Asthma Immunol 117:595–600. https://doi.org/10.1016/j.anai.2016.07.043
Wolfgram PM, Allen DB (2017) Effects of inhaled corticosteroids on growth, bone metabolism, and adrenal function. Adv Pediatr 64:331–345. https://doi.org/10.1016/j.yapd.2017.03.006
van Staa TP, Bishop N, Leufkens HG, Cooper C (2004) Are inhaled corticosteroids associated with an increased risk of fracture in children? Osteoporos Int 15:785–791. https://doi.org/10.1007/s00198-004-1606-5
Ward LM (2021) Part I: Which child with a chronic disease needs bone health monitoring? Curr Osteoporos Rep 19:278–288. https://doi.org/10.1007/s11914-021-00667-x
Formosa MM, Bergen DJM, Gregson CL, Maurizi A, Kampe A, Garcia-Giralt N, Zhou W, Grinberg D, Crespo DO, Zillikens MC, Williams GR, Bassett JHD, Brandi ML, Sangiorgi L, Balcells S, Hogler W, Van Hul W, Makitie O (2021) A roadmap to gene discoveries and novel therapies in monogenic low and high bone mass disorders. Front Endocrinol 12:709711. https://doi.org/10.3389/fendo.2021.709711
Binkovitz LA, Henwood MJ (2007) Pediatric DXA: Technique and interpretation. Pediatr Radiol 37:21–31. https://doi.org/10.1007/s00247-006-0153-y
Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, Fuleihan GEH, Kecskemethy HH, Jaworski M, Gordon CM, International Society for Clinical D (2014) Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 17:225–242. https://doi.org/10.1016/j.jocd.2014.01.003
Kalkwarf HJ, Abrams SA, DiMeglio LA, Koo WW, Specker BL, Weiler H (2014) Bone densitometry in infants and young children: the 2013 ISCD Pediatric Official Positions. J Clin Densitom 17:243–257. https://doi.org/10.1016/j.jocd.2014.01.002
Bachrach LK, Gordon CM, Section OE (2016) Bone densitometry in children and adolescents. Pediatrics. https://doi.org/10.1542/peds.2016-2398
Mergler S, de Man SA, Boot AM, Heus KG, Huijbers WA, van Rijn RR, Penning C, Evenhuis HM (2016) Automated radiogrammetry is a feasible method for measuring bone quality and bone maturation in severely disabled children. Pediatr Radiol 46:1017–1022. https://doi.org/10.1007/s00247-016-3548-4
Jorgensen JT, Andersen PB, Rosholm A, Hannover BN (2000) Digital X-ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Physiol 20:330–335. https://doi.org/10.1046/j.1365-2281.2000.00268.x
Rosholm A, Hyldstrup L, Bæksgaard L, Grunkin M, Thodberg HH (2001) Estimation of bone mineral density by digital X-ray radiogrammetry: Theoretical background and clinical testing. Osteoporos Int 12:961–969. https://doi.org/10.1007/s001980170026
Leijten AD, Hampsink B, Janssen M, Klein WM, Draaisma JMT (2019) Can digital X-ray radiogrammetry be an alternative for dual-energy X-ray absorptiometry in the diagnosis of secondary low bone quality in children? Eur J Pediatr 178:1433–1441. https://doi.org/10.1007/s00431-019-03425-5
Schundeln MM, Marschke L, Bauer JJ, Hauffa PK, Schweiger B, Fuhrer-Sakel D, Lahner H, Poeppel TD, Kiewert C, Hauffa BP, Grasemann C (2016) A piece of the puzzle: the bone health index of the BoneXpert software reflects cortical bone mineral density in pediatric and adolescent patients. PLoS ONE 11:e0151936. https://doi.org/10.1371/journal.pone.0151936
Alshamrani K, Messina F, Bishop N, Offiah AC (2019) Estimating bone mass in children: Can bone health index replace dual energy x-ray absorptiometry? Pediatr Radiol 49:372–378. https://doi.org/10.1007/s00247-018-4309-3
van Rijn RR, Boot A, Wittenberg R, van der Sluis IM, van den Heuvel-Eibrink MM, Lequin MH, de Muinck Keizer-Schrama SM, Van Kuijk C (2006) Direct X-ray radiogrammetry versus dual-energy X-ray absorptiometry: Assessment of bone density in children treated for acute lymphoblastic leukaemia and growth hormone deficiency. Pediatr Radiol 36:227–232. https://doi.org/10.1007/s00247-005-0080-3
Mentzel HJ, Blume J, Boettcher J, Lehmann G, Tuchscherer D, Pfeil A, Kramer A, Malich A, Kauf E, Hein G, Kaiser WA (2006) The potential of digital X-ray radiogrammetry (DXR) in the assessment of osteopenia in children with chronic inflammatory bowel disease. Pediatr Radiol 36:415–420. https://doi.org/10.1007/s00247-005-0093-y
Neelis E, Rijnen N, Sluimer J, Olieman J, Rizopoulos D, Wijnen R, Rings E, de Koning B, Hulst J (2018) Bone health of children with intestinal failure measured by dual energy X-ray absorptiometry and digital X-ray radiogrammetry. Clin Nutr 37:687–694. https://doi.org/10.1016/j.clnu.2017.02.014
Nusman CM, Anink J, Otten MH, van Rossum MA, van Rijn RR, Maas M, van Suijlekom-Smit LW (2015) Bone health of patients with juvenile idiopathic arthritis: a comparison between dual-energy X-ray absorptiometry and digital X-ray radiogrammetry. Eur J Radiol 84:1999–2003. https://doi.org/10.1016/j.ejrad.2015.06.015
Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP (2015) The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum 44:571–585. https://doi.org/10.1016/j.semarthrit.2014.10.006
Alqahtani FF, Offiah AC (2019) Diagnosis of osteoporotic vertebral fractures in children. Pediatr Radiol 49:283–296. https://doi.org/10.1007/s00247-018-4279-5
Genant HK, Wu CY, Van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Wang YXJ, Santiago FR, Deng M, Nogueira-Barbosa MH (2017) Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg 7:555–591. https://doi.org/10.21037/qims.2017.10.05
Grigoryan M, Guermazi A, Roemer FW, Delmas PD, Genant HK (2003) Recognizing and reporting osteoporotic vertebral fractures. Eur Spine J 12(Suppl 2):S104-112. https://doi.org/10.1007/s00586-003-0613-0
Crabtree NJ, Chapman S, Hogler W, Hodgson K, Chapman D, Bebbington N, Shaw NJ (2017) Vertebral fractures assessment in children: evaluation of DXA imaging versus conventional spine radiography. Bone 97:168–174. https://doi.org/10.1016/j.bone.2017.01.006
Weber DR, Boyce A, Gordon C, Hogler W, Kecskemethy HH, Misra M, Swolin-Eide D, Tebben P, Ward LM, Wasserman H, Shuhart C, Zemel BS (2019) The utility of DXA assessment at the forearm, proximal femur, and lateral distal femur, and vertebral fracture assessment in the pediatric population: 2019 ISCD official position. J Clin Densitom 22:567–589. https://doi.org/10.1016/j.jocd.2019.07.002
Malgo F, Hamdy NAT, Ticheler C, Smit F, Kroon HM, Rabelink TJ, Dekkers OM, Appelman-Dijkstra NM (2017) Value and potential limitations of vertebral fracture assessment (VFA) compared to conventional spine radiography: Experience from a fracture liaison service (FLS) and a meta-analysis. Osteoporos Int 28:2955–2965. https://doi.org/10.1007/s00198-017-4137-6
Zemel BS (2011) Quantitative computed tomography and computed tomography in children. Curr Osteoporos Rep 9:284–290. https://doi.org/10.1007/s11914-011-0076-x
Rauch F, Schönau E (2008) Peripheral quantitative computed tomography of the proximal radius in young subjects – new reference data and interpretation of results. J Musculoskelet Neuronal Interact 8:217–226
Rauch F, Schönau E (2005) Peripheral quantitative computed tomography of the distal radius in young subjects – new reference data and interpretation of results. J Musculoskelet Neuronal Interact 5
Jaworski M, Kobylińska M, Graff K (2021) Peripheral quantitative computed tomography of the lower leg in children and adolescents: Bone densities, cross-sectional sizes and muscle distribution reference data. J Musculoskelet Neuronal Interact 21:215–236
Lalayiannis AD, Fewtrell M, Biassoni L, Silva S, Goodman N, Shroff R, Crabtree NJ (2021) Studying bone mineral density in young people: the complexity of choosing a pQCT reference database. Bone 143:115713. https://doi.org/10.1016/j.bone.2020.115713
Di Iorgi N, Maruca K, Patti G, Mora S (2018) Update on bone density measurements and their interpretation in children and adolescents. Best Pract Res Clin Endocrinol Metab 32:477–498. https://doi.org/10.1016/j.beem.2018.06.002
Baroncelli GI (2008) Quantitative ultrasound methods to assess bone mineral status in children: Technical characteristics, performance, and clinical application. Pediatr Res 63:220–228. https://doi.org/10.1203/PDR.0b013e318163a286
Baroncelli GI, Federico G, Vignolo M, Valerio G, Del Puente A et al (2006) Cross-sectional reference data for phalangeal quantitative ultrasound from early childhood to young-adulthood according to gender, age, skeletal growth, and pubertal development. Bone 39:159–173. https://doi.org/10.1016/j.bone.2005.12.010
Adamczyk P, Szczepanska M, Pluskiewicz W (2018) Skeletal status assessment by quantitative ultrasound and bone densitometry in children with different renal conditions. Osteoporos Int 29:2667–2675. https://doi.org/10.1007/s00198-018-4659-6
Chong KH, Poh BK, Jamil NA, Kamaruddin NA, Deurenberg P (2015) Radial quantitative ultrasound and dual energy x-ray absorptiometry: Intermethod agreement for bone status assessment in children. Biomed Res Int 2015:232876. https://doi.org/10.1155/2015/232876
Mayranpaa MK, Tamminen IS, Kroger H, Makitie O (2011) Bone biopsy findings and correlation with clinical, radiological, and biochemical parameters in children with fractures. J Bone Miner Res 26:1748–1758. https://doi.org/10.1002/jbmr.373
Funding
No founding to declare.
Author information
Authors and Affiliations
Contributions
SC and JSR contributed to the conception and design of the manuscript. SC, TCJS and JSR edited the manuscript. All the authors contributed to the literature search, revised the article and approved its final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable, radiographs and DXA scan results are completely anonymized.
Conflicts of interest
The authors have no conflict of interests to declare.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciancia, S., van Rijn, R.R., Högler, W. et al. Osteoporosis in children and adolescents: when to suspect and how to diagnose it. Eur J Pediatr 181, 2549–2561 (2022). https://doi.org/10.1007/s00431-022-04455-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04455-2