Abstract
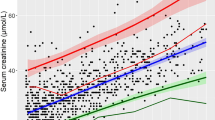
Standard serum creatinine (S–Cr) levels in healthy children fluctuate with age and sex. However, it is unclear if this fluctuation in S–Cr levels is present for children with Down syndrome (DS) who show atypical growth rate. Therefore, we aimed to establish specific reference S–Cr levels for DS and compare them with the prevailing standard levels. We retrospectively reviewed 984 children with DS aged 3 months to 18 years who visited our medical center. Patients with diseases affecting S–Cr levels were excluded. We calculated the reference S–Cr levels according to sex, age, and length/height using medical records. A total of 3765 examinations of 568 children with DS were registered for this study. Ages and S–Cr levels were examined for boys (y = 0.032x + 0.20; r = 0.868, P < 0.0001), and girls (y = 0.024x + 0.23; r = 0.835, P < 0.0001). S–Cr levels in children aged >9 years were significantly higher in boys than in girls. The 430 children with DS aged 2–8 years were examined 1867 times. Height and S–Cr levels showed a significantly strong positive correlation (r = 0.670, P < 0.001) with regression equation y = 0.37x. The quintic equations calculated with S–Cr levels and length/height for boys (336 children, 2043 tests, r = 0.887) and girls (232 children, 1722 tests, r = 0.805) werey = − 6.132x5 + 32.78x4 − 67.86x3 + 68.31x2 − 33.14x + 6.41, and y = 0.09542x5 + 1.295x4 − 6.401x3 + 10.35x2 − 6.746x + 1.772. All calculated results varied from the standard levels for healthy children.
Conclusion: This study established reference S–Cr levels and quintic equations specific for children with DS. These reference levels would be potentially useful in evaluating S–Cr levels and renal function in this population.
What is Known: •Standard serum creatinine levels vary with age and sex to reflect muscle mass. •Reference serum creatinine levels specific to children with Down syndrome who show growth rates different from those of healthy children have not been established. | |
What is New: •Serum creatinine levels in children with Down syndrome showed different trajectories for sex, age, and length/height when compared with the standard levels for healthy children. •This report on specific reference serum creatinine levels for children with Down syndrome is useful in the assessment of renal function in these children. |

Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Abbreviations
- ASD:
-
Atrial septal defect
- AVSD:
-
Atrioventricular septal defect
- CAKUT:
-
Congenital anomalies of the kidney and urinary tract
- CGN:
-
Chronic glomerulonephritis
- CHD:
-
Congenital heart disease
- DORV:
-
Double-outlet right ventricle
- DS:
-
Down syndrome
- MAE:
-
Mean absolute error
- ME:
-
Mean error
- PDA:
-
Patent ductus arteriosus
- PFO:
-
Patent foramen ovale
- RMSE:
-
Root mean square error
- S–Cr:
-
Serum creatinine
- TOF:
-
Tetralogy of Fallot
- VSD:
-
Ventricular septal defect
- VUR:
-
Vesicoureteral reflux
References
Schwartz GJ, Haycock GB, Spitzer A (1976) Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr 88:828–830
Savory DJ (1990) Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem 27(Pt 2):99–101
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Kitagawa T (2011) Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol 15:694–699
Zemel BS, Pipan M, Stallings VA, Hall W, Schadt K, Freedman DS, Thorpe P (2015) Growth charts for children with Down syndrome in the United States. Pediatrics 136:e1204–e1211
Hatch-Stein JA, Zemel BS, Prasad D, Kalkwarf HJ, Pipan M, Magge SN, Kelly A (2016) Body composition and BMI growth charts in children with Down syndrome. Pediatrics 138:e20160541
Hook EB, Cross PK (1982) Paternal age and Down’s syndrome genotypes diagnosed prenatally: no association in New York state data. Hum Genet 62:167–174
Mutton D, Alberman E, Hook EB (1996) Cytogenetic and epidemiological findings in Down syndrome, England and Wales 1989 to 1993. National Down Syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J Med Genet 33:387–394
Papavassiliou P, Charalsawadi C, Rafferty K, Jackson-Cook C (2015) Mosaicism for trisomy 21: a review. Am J Med Genet A 167A:26–39
Bull MJ, Committee on G (2011) Health supervision for children with Down syndrome. Pediatrics 128:393–406
Capone GT, Chicoine B, Bulova P, Stephens M, Hart S, Crissman B, Videlefsky A, Myers K, Roizen N, Esbensen A, Peterson M, Santoro S, Woodward J, Martin B, Smith D, Down Syndrome Medical Interest Group D-USAAHCW (2018) Co-occurring medical conditions in adults with Down syndrome: a systematic review toward the development of health care guidelines. Am J Med Genet A 176:116–133
Malaga S, Pardo R, Malaga I, Orejas G, Fernandez-Toral J (2005) Renal involvement in Down syndrome. Pediatr Nephrol 20:614–617
Nishino T, Endo S, Miyano H, Umeda C, Tomii Y, Watanabe Y, Nakagawa M, Kakegawa D, Fujinaga S (2020) Is the eGFR formula useful for evaluating the renal function of Down syndrome? Pediatr Int. https://doi.org/10.1111/ped.14539
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Daschner M (2005) Drug dosage in children with reduced renal function. Pediatr Nephrol 20:1675–1686
Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almen T, Aspelin P, Bellin MF, Clement O, Heinz-Peer G, Contrast Media Safety Committee of European Society of Urogenital R (2011) Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 21:2527–2541
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843
Subrahmanyam AB, Mehta AV (1995) Renal anomalies in Down syndrome. Pediatr Nephrol 9:253–254
Kallen B, Mastroiacovo P, Robert E (1996) Major congenital malformations in Down syndrome. Am J Med Genet 65:160–166
Niamien-Attai C, Bacchetta J, Ranchin B, Sanlaville D, Cochat P (2017) Renal abnormalities in Down syndrome: A review. Arch Pediatr 24:1013–1018
Stoll C, Dott B, Alembik Y, Roth MP (2015) Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet 58:674–680
Rankin J, Tennant PW, Bythell M, Pearce MS (2012) Predictors of survival in children born with Down syndrome: a registry-based study. Pediatrics 129:e1373–e1381
Morris JK, Garne E, Wellesley D, Addor MC, Arriola L, Barisic I, Beres J, Bianchi F, Budd J, Dias CM, Gatt M, Klungsoyr K, Khoshnood B, Latos-Bielenska A, Mullaney C, Nelen V, Neville AJ, O'Mahony M, Queisser-Luft A, Randrianaivo H, Rankin J, Rissmann A, Rounding C, Sipek A, Stoianova S, Tucker D, de Walle H, Yevtushok L, Loane M, Dolk H (2014) Major congenital anomalies in babies born with Down syndrome: a EUROCAT population-based registry study. Am J Med Genet A 164A:2979–2986
Kupferman JC, Druschel CM, Kupchik GS (2009) Increased prevalence of renal and urinary tract anomalies in children with Down syndrome. Pediatrics 124:e615–e621
Toth R, Szanto P, Prodan Z, Lex DJ, Sapi E, Szatmari A, Gal J, Szanto T, Szekely A (2013) Down syndrome and postoperative complications after paediatric cardiac surgery: a propensity-matched analysis. Interact Cardiovasc Thorac Surg 17:691–697
Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR, Consortium T-A (2016) Kidney outcomes 5 years after pediatric cardiac surgery: the TRIBE-AKI study. JAMA Pediatr 170:1071–1078
Huynh L, Rodriguez-Lopez S, Benisty K, Dancea A, Garros D, Hessey E, Joffe A, Joffe R, Mackie A, Palijan A, Paun A, Pizzi M, Zappitelli M, Morgan C (2020) Follow-up after neonatal heart disease repair: watch out for chronic kidney disease and hypertension! Pediatr Nephrol 35:2137–2145
Beyer C (1993) Creatine measurement in serum and urine with an automated enzymatic method. Clin Chem 39:1613–1619
Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, National Kidney Disease Education Program Laboratory Working G (2006) Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52:5–18
Agamah ES, Webber LS, Lawrence M, Wattigney W, Berenson GS (1990) Serum creatinine and its relation to cardiovascular disease risk variables in children and young adults from a biracial community. The Bogalusa Heart Study. J Lab Clin Med 116:327–334
Groesbeck D, Kottgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, Furth S (2008) Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol 3:1777–1785
Liu C, Wen J, Xiang J, Ouyang X, Yang Y, Lu W, Wang J, Huang J, Min X (2019) Age- and sex-specific reference intervals for the serum cystatin C/creatinine ratio in healthy children (0-18 years old). J Int Med Res 47:3151–3159
Ghasemi A, Azimzadeh I, Afghan M, Momenan AA, Bagheripour F, Azizi F (2015) Pediatric reference values for serum creatinine and estimated glomerular filtration rate in Iranians: Tehran Lipid and Glucose Study. Arch Iran Med 18:753–759
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Acknowledgements
We would like to thank Dr. Uemura for his kind advice.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
Tomohiko Nishino mainly drafted the manuscript and performed the statistical analysis; Tomohiko Nishino, Shota Endo, Hiroki Miyano, Yoichi Takemasa, Masahito Saito, Chisato Umeda, and Yuji Tomii contributed to the conception and design of this study; Yoshitaka Watanabe, Mayu Nakagawa, and Daisuke Kakegawa critically reviewed the manuscript; Shuichiro Fujinaga supervised the whole study process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee for human research (protocol number 2020-04-025). All procedures involving human participants were conducted according to the ethical standards of the institutional and/or national research committee and within the guidelines of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent was waived by the institutional review board due to the retrospective nature of the study.
Consent for publication
In consideration of human rights and ethics, this research was conducted under the review of the ethics committee of the medical center, and the study plan outline was presented on our center’s website, where patients and parents could ask questions about the study and opt-out from the use of their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Gregorio Paolo Milani
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishino, T., Endo, S., Miyano, H. et al. Reference serum creatinine levels according to sex, age, and height in children with Down syndrome. Eur J Pediatr 180, 2977–2983 (2021). https://doi.org/10.1007/s00431-021-04078-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04078-z