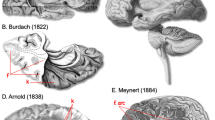
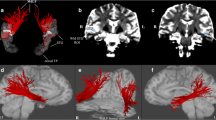
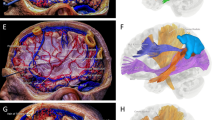
Abstract
The angular gyrus (AG) has been described in numerous studies to be consistently activated in various functional tasks. The angular gyrus is a critical connector epicenter linking multiple functional networks due to its location in the posterior part of the inferior parietal cortex, namely at the junction between the parietal, temporal, and occipital lobes. It is thus crucial to identify the different pathways that anatomically connect this high-order association region to the rest of the brain. Our study revisits the three-dimensional architecture of the structural AG connectivity by combining state-of-the-art postmortem blunt microdissection with advanced in vivo diffusion tractography to comprehensively describe the association, projection, and commissural fibers that connect the human angular gyrus. AG appears as a posterior “angular stone” of associative connections belonging to mid- and long-range dorsal and ventral fibers of the superior and inferior longitudinal systems, respectively, to short-range parietal, occipital, and temporal fibers, including U-shaped fibers in the posterior transverse system. Thus, AG is at a pivotal dorso-ventral position reflecting its critical role in the different functional networks, particularly in language elaboration and spatial attention and awareness in the left and right hemispheres, respectively. We also reveal striatal, thalamic, and brainstem connections and a typical inter-hemispheric homotopic callosal connectivity supporting the suggested AG role in the integration of sensory input for modulating motor control and planning. The present description of AG’s highly distributed wiring diagram may drastically improve intraoperative subcortical testing and post-operative neurologic outcomes related to surgery in and around the angular gyrus.











Similar content being viewed by others
Data availability
The in vivo diffusion MRI dataset analyzed in the current study belongs to the BIL&GIN database. The BIL&GIN is not freely available, but a data-sharing model based on collaborative research agreements has been implemented. Request for joint research projects can be made through the BIL&GIN website or by email to the corresponding author of the present paper.
Code availability
Software application or custom code: no specific code developed, not applicable.
References
Avants BB, Tustison NJ, Song G (2009) Advanced normalization tools (ANTS). Insight J 2:1–35
Box GEP, Cox DR (1964) An analysis of transformations. J Roy Stat Soc Ser B (methodol) 26(2):211–252
Bullock D, Takemura H, Caiafa CF, Kitchell L, McPherson B, Caron B, Pestilli F (2019) Associative white matter connecting the dorsal and ventral posterior human cortex. Brain Struct Funct 224(8):2631–2660. https://doi.org/10.1007/s00429-019-01907-8
Catani M, Robertsson N, Beyh A, Huynh V, de Santiago RF, Howells H, Barrett RLC, Aiello M, Cavaliere C, Dyrby TB, Krug K, Ptito M, D’Arceuil H, Forkel SJ, Dell’Acqua F (2017) Short parietal lobe connections of the human and monkey brain. Cortex 97:339–357. https://doi.org/10.1016/j.cortex.2017.10.022
Cattaneo L, Barchiesi G (2011) Transcranial magnetic mapping of the short-latency modulations of corticospinal activity from the ipsilateral hemisphere during rest. Front Neural Circuits 5:14. https://doi.org/10.3389/fncir.2011.00014
Cattaneo L, Giampiccolo D, Meneghelli P, Tramontano V, Sala F (2020) Cortico-cortical connectivity between the superior and inferior parietal lobules and the motor cortex assessed by intraoperative dual cortical stimulation. Brain Stimul 13(3):819–831. https://doi.org/10.1016/j.brs.2020.02.023
Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG (2014) Q-Ball of Inferior Fronto-Occipital Fasciculus and Beyond. PLoS ONE 9(6):e100274. https://doi.org/10.1371/journal.pone.0100274
Chenot Q, Tzourio-Mazoyer N, Rheault F, Descoteaux M, Crivello F, Zago L, Mellet E, Jobard G, Joliot M, Mazoyer B, Petit L (2019) A population-based atlas of the human pyramidal tract in 410 healthy participants. Brain Struct Funct 224(2):599–612. https://doi.org/10.1007/s00429-018-1798-7
Crosby E, Humphrey T, Lauer EW (1962) Correlative anatomy of the nervous system. The MacMillan Company, New York
De Benedictis A, Petit L, Descoteaux M, Marras CE, Barbareschi M, Corsini F, Dallabona M, Chioffi F, Sarubbo S (2016) New insights in the homotopic and heterotopic connectivity of the frontal portion of the human corpus callosum revealed by microdissection and diffusion tractography. Hum Brain Mapp 37(12):4718–4735. https://doi.org/10.1002/hbm.23339
De Benedictis A, Nocerino E, Menna F, Remondino F, Barbareschi M, Rozzanigo U, Corsini F, Olivetti E, Marras CE, Chioffi F, Avesani P, Sarubbo S (2018) Photogrammetry of the human brain: a novel method for three-dimensional quantitative exploration of the structural connectivity in neurosurgery and neurosciences. World Neurosurg 115:e279–e291. https://doi.org/10.1016/j.wneu.2018.04.036
De Benedictis A, Marras CE, Petit L, Sarubbo S (2021) The inferior fronto-occipital fascicle: a century of controversies from anatomy theaters to operative neurosurgery. J Neurosurg Sci 65(6):605–615. https://doi.org/10.23736/S0390-5616.21.05360-1
Dejerine J, Dejerine-Klumpke A (1895) Anatomie des centres nerveux. Tome 1. Rueff et Cie, Paris
Dejerine J, Dejerine-Klumpke A (1901) Anatomie des centres nerveux. Tome 2. Rueff et Cie, Paris
Di Carlo DT, Benedetto N, Duffau H, Cagnazzo F, Weiss A, Castagna M, Cosottini M, Perrini P (2019) Microsurgical anatomy of the sagittal stratum. Acta Neurochir (wien) 161(11):2319–2327. https://doi.org/10.1007/s00701-019-04019-8
Di Tommaso P, Chatzou M, Floden EW, Barja PP, Palumbo E, Notredame C (2017) Nextflow enables reproducible computational workflows. Nat Biotechnol 35(4):316–319. https://doi.org/10.1038/nbt.3820
Gallivan JP, Culham JC (2015) Neural coding within human brain areas involved in actions. Curr Opin Neurobiol 33:141–149. https://doi.org/10.1016/j.conb.2015.03.012
Guevara M, Guevara P, Román C, Mangin J-F (2020) Superficial white matter: a review on the dMRI analysis methods and applications. Neuroimage 212:116673. https://doi.org/10.1016/j.neuroimage.2020.116673
Hau J, Sarubbo S, Perchey G, Crivello F, Zago L, Mellet E, Jobard G, Joliot M, Mazoyer BM, Tzourio-Mazoyer N, Petit L (2016) Cortical terminations of the inferior fronto-occipital and uncinate fasciculi: anatomical stem-based virtual dissection. Front Neuroanat 10:58. https://doi.org/10.3389/fnana.2016.00058
Jiao Y, Lin F, Wu J, Li H, Fu W, Huo R, Cao Y, Wang S, Zhao J (2020) Plasticity in language cortex and white matter tracts after resection of dominant inferior parietal lobule arteriovenous malformations: a combined fMRI and DTI study. J Neurosurg 134(3):953–960. https://doi.org/10.3171/2019.12.JNS191987
Kinomura S, Larsson J, Gulyas B, Roland PE (1996) Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271:512–515
Kiriyama I, Miki H, Kikuchi K, Ohue S, Matsuda S, Mochizuki T (2009) Topographic analysis of the inferior parietal lobule in high-resolution 3D MR imaging. AJNR Am J Neuroradiol 30(3):520–524. https://doi.org/10.3174/ajnr.A1417
Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, Rothwell J, Bozzali M (2010) In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuroimage 51(1):300–312. https://doi.org/10.1016/j.neuroimage.2010.02.022
Kurtzer GM, Sochat V, Bauer MW (2017) Singularity: Scientific containers for mobility of compute. PLoS ONE 12(5):e0177459. https://doi.org/10.1371/journal.pone.0177459
LaBerge D (1995) Computational and anatomical models of selective attention in object identification. In: Gazzaniga MS (ed) The cognitive neurosciences, vol 41. MIT Press, Cambridge, pp 649–663
Latini F, Trevisi G, Fahlstrom M, Jemstedt M, Alberius Munkhammar A, Zetterling M, Hesselager G, Ryttlefors M (2020) New insights into the anatomy, connectivity and clinical implications of the middle longitudinal fasciculus. Front Neuroanat 14(106):610324. https://doi.org/10.3389/fnana.2020.610324
Ludwig E, Klingler J (1956) Atlas cerebri humani. S. Karger, Basel
Maffei C, Sarubbo S, Jovicich J (2019) Diffusion-based tractography atlas of the human acoustic radiation. Sci Rep 9(1):4046. https://doi.org/10.1038/s41598-019-40666-8
Mahdy Ali K, Avesani P (2021) The vertical superior longitudinal fascicle and the vertical occipital fascicle. J Neurosurg Sci 65(6):581–589. https://doi.org/10.23736/S0390-5616.21.05368-6
Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, Chamberland M, Yeh FC, Lin YC, Ji Q, Reddick WE, Glass JO, Chen DQ, Feng Y, Gao C, Wu Y, Ma J, He R, Li Q, Westin CF, Deslauriers-Gauthier S, Gonzalez JOO, Paquette M, St-Jean S, Girard G, Rheault F, Sidhu J, Tax CMW, Guo F, Mesri HY, David S, Froeling M, Heemskerk AM, Leemans A, Bore A, Pinsard B, Bedetti C, Desrosiers M, Brambati S, Doyon J, Sarica A, Vasta R, Cerasa A, Quattrone A, Yeatman J, Khan AR, Hodges W, Alexander S, Romascano D, Barakovic M, Auria A, Esteban O, Lemkaddem A, Thiran JP, Cetingul HE, Odry BL, Mailhe B, Nadar MS, Pizzagalli F, Prasad G, Villalon-Reina JE, Galvis J, Thompson PM, Requejo FS, Laguna PL, Lacerda LM, Barrett R, Dell’Acqua F, Catani M, Petit L, Caruyer E, Daducci A, Dyrby TB, Holland-Letz T, Hilgetag CC, Stieltjes B, Descoteaux M (2017) The challenge of mapping the human connectome based on diffusion tractography. Nat Commun 8(1):1349. https://doi.org/10.1038/s41467-017-01285-x
Makris N, Preti MG, Wassermann D, Rathi Y, Papadimitriou GM, Yergatian C, Dickerson BC, Shenton ME, Kubicki M (2013) Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging Behav 7(3):335–352. https://doi.org/10.1007/s11682-013-9235-2
Makris N, Zhu A, Papadimitriou GM, Mouradian P, Ng I, Scaccianoce E, Baselli G, Baglio F, Shenton ME, Rathi Y, Dickerson B, Yeterian E, Kubicki M (2017) Mapping temporo-parietal and temporo-occipital cortico-cortical connections of the human middle longitudinal fascicle in subject-specific, probabilistic, and stereotaxic Talairach spaces. Brain Imaging Behav 11(5):1258–1277. https://doi.org/10.1007/s11682-016-9589-3
Mandonnet E, Sarubbo S, Petit L (2018) The nomenclature of human white matter association pathways: proposal for a systematic taxonomic anatomical classification. Front Neuroanat 12:94. https://doi.org/10.3389/fnana.2018.00094
Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2010) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46(5):691–699. https://doi.org/10.1016/j.cortex.2009.07.015
Matsumoto R, Nair DR, Ikeda A, Fumuro T, LaPresto E, Mikuni N, Bingaman W, Miyamoto S, Fukuyama H, Takahashi R, Najm I, Shibasaki H, Lüders HO (2012) Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum Brain Mapp 33(12):2856–2872. https://doi.org/10.1002/hbm.21407
Mazoyer B, Mellet E, Perchey G, Zago L, Crivello F, Jobard G, Delcroix N, Vigneau M, Leroux G, Petit L, Joliot M, Tzourio-Mazoyer N (2016) BIL&GIN: A neuroimaging, cognitive, behavioral, and genetic database for the study of human brain lateralization. Neuroimage. https://doi.org/10.1016/j.neuroimage.2015.02.071
Mesulam M-M (2008) Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann Neurol 64(4):367–378
Meynert T (1885) Psychiatry: clinical treatise on the diseases of the fore-brain, trans. B. Sachs. GP Putnam, New York & London
Nieuwenhuys R, Voogd J, van Huijzen C (2008) The human central nervous system, 4th edn. Springer-Verlag, Berlin
Niu M, Palomero-Gallagher N (2022) Architecture and connectivity of the human angular gyrus and of its homolog region in the macaque brain. Brain Struct Funct. https://doi.org/10.1007/s00429-022-02509-7
Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PCM, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S (2009) Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 46(2):486–499
Palejwala AH, O’Connor KP, Pelargos P, Briggs RG, Milton CK, Conner AK, Milligan TM, O’Donoghue DL, Glenn CA, Sughrue ME (2020) Anatomy and white matter connections of the lateral occipital cortex. Surg Radiol Anat 42(3):315–328. https://doi.org/10.1007/s00276-019-02371-z
Petit L, Rheault F, Descoteaux M, Tzourio-Mazoyer N Half of the streamlines built in a whole human brain tractogram is anatomically uninterpretable. In: 25th Conference of the Organization for Human Brain Mapping, Roma, 2019.
Petit L, Pouget P (2019) The comparative anatomy of frontal eye fields in primates. Cortex 118:51–64. https://doi.org/10.1016/j.cortex.2019.02.023
Rebelo D, Oliveira F, Abrunhosa A, Januario C, Lemos J, Castelo-Branco M (2021) A link between synaptic plasticity and reorganization of brain activity in Parkinson’s disease. Proc Natl Acad Sci U S A 118(3):e2013962118. https://doi.org/10.1073/pnas.2013962118
Rheault F, Poulin P, Valcourt Caron A, St-Onge E, Descoteaux M (2020) Common misconceptions, hidden biases and modern challenges of dMRI tractography. J Neural Eng 17(1):011001. https://doi.org/10.1088/1741-2552/ab6aad
Richard N, Desmurget M, Teillac A, Beuriat PA, Bardi L, Coude G, Szathmari A, Mottolese C, Sirigu A, Hiba B (2021) Anatomical bases of fast parietal grasp control in humans: a diffusion-MRI tractography study. Neuroimage 235:118002. https://doi.org/10.1016/j.neuroimage.2021.118002
Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G (2006) Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex 16(10):1389–1417. https://doi.org/10.1093/cercor/bhj076
Sarubbo S, Le Bars E, Moritz-Gasser S, Duffau H (2012) Complete recovery after surgical resection of left Wernicke’s area in awake patient: a brain stimulation and functional MRI study. Neurosurg Rev 35(2):287–292. https://doi.org/10.1007/s10143-011-0351-4
Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H (2013) Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct 218:21–37. https://doi.org/10.1007/s00429-011-0372-3
Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Balbi S, Granieri E, Duffau H (2015) Towards a functional atlas of human white matter. Hum Brain Mapp 36(8):3117–3136. https://doi.org/10.1002/hbm.22832
Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Barbareschi M, Dallabona M, Chioffi F, Duffau H (2016) Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37(11):3858–3872. https://doi.org/10.1002/hbm.23281
Sarubbo S, Tate M, De Benedictis A, Merler S, Moritz-Gasser S, Herbet G, Duffau H (2020) Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205:116237. https://doi.org/10.1016/j.neuroimage.2019.116237
Schilling KG, Tax CMW, Rheault F, Landman BA, Anderson AW, Descoteaux M, Petit L (2022) Prevalence of white matter pathways coming into a single white matter voxel orientation: the bottleneck issue in tractography. Hum Brain Mapp 43(4):1196–1213. https://doi.org/10.1002/hbm.25697
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, New York
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19(1):43–61. https://doi.org/10.1177/1073858412440596
Sparing R, Thimm M, Hesse MD, Kust J, Karbe H, Fink GR (2009) Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain 132(Pt 11):3011–3020. https://doi.org/10.1093/brain/awp154
Theaud G, Houde JC, Bore A, Rheault F, Morency F, Descoteaux M (2020) TractoFlow: a robust, efficient and reproducible diffusion mri pipeline leveraging nextflow & singularity. Neuroimage 218:116889. https://doi.org/10.1016/j.neuroimage.2020.116889
Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P (2005) Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309(5744):2226–2228
Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM, Catani M (2011) A lateralized brain network for visuospatial attention. Nat Neurosci 14(10):1245–1246
Vavassori L, Sarubbo S, Petit L (2021) Hodology of the superior longitudinal system of the human brain: a historical perspective, the current controversies, and a proposal. Brain Struct Funct 226(5):1363–1384. https://doi.org/10.1007/s00429-021-02265-0
Vigneau M, Beaucousin V, Hervé P-Y, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30(4):1414–1432
Wang Y, Fernandez-Miranda JC, Verstynen T, Pathak S, Schneider W, Yeh F-C (2013) Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex 23(10):2347–2356. https://doi.org/10.1093/cercor/bhs225
Wang X, Pathak S, Stefaneanu L, Yeh FC, Li S, Fernandez-Miranda JC (2016) Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct 221(4):2075–2092. https://doi.org/10.1007/s00429-015-1028-5
Wu Y, Sun D, Wang Y, Wang Y (2016a) Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Front Neuroanat 10:88. https://doi.org/10.3389/fnana.2016.00088
Wu Y, Sun D, Wang Y, Wang Y, Wang Y (2016b) Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res 1646:152–159. https://doi.org/10.1016/j.brainres.2016.05.046
Xu Y, He Y, Bi Y (2017) A Tri-network Model of Human Semantic Processing. Front Psychol 8:1538. https://doi.org/10.3389/fpsyg.2017.01538
Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA (2014) The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci U S A 111(48):E5214-5223. https://doi.org/10.1073/pnas.1418503111
Yeh FC (2020) Shape analysis of the human association pathways. Neuroimage 223:117329. https://doi.org/10.1016/j.neuroimage.2020.117329
Yeterian EH, Pandya DN (1993) Striatal connections of the parietal assoiation cortices in rhesus monkey. JCompNeurol 332:175–197
Zaca D, Corsini F, Rozzanigo U, Dallabona M, Avesani P, Annicchiarico L, Zigiotto L, Faraca G, Chioffi F, Jovicich J, Sarubbo S (2018) Whole-brain network connectivity underlying the human speech articulation as emerged integrating direct electric stimulation, resting state fMRI and tractography. Front Hum Neurosci 12:405. https://doi.org/10.3389/fnhum.2018.00405
Zemmoura I, Blanchard E, Raynal PI, Rousselot-Denis C, Destrieux C, Velut S (2016) How Klingler’s dissection permits exploration of brain structural connectivity? An electron microscopy study of human white matter. Brain Struct Funct 221(5):2477–2486. https://doi.org/10.1007/s00429-015-1050-7
Funding
No particular funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
No conflict of interest for any authors.
Ethical approval
The present manuscript is not submitted elsewhere for simultaneous consideration; the submitted work is original and has not been published elsewhere in any form or language (partially or in whole); no re-use of material to avoid the concerns about text-recycling (self-plagiarism) in the present manuscript; the part of the study, including in vivo diffusion MRI data, was approved by the local ethics committee (CCPRB Basse-Normandie, France).The part of the study, including postmortem dissection data, was approved by the Ethical Committee of the Azienda Provinciale per i Servizi Sanitari, Trento, Italy (N° 06/2018).
Informed consent
For the part of the study involving in vivo diffusion MRI data, all participants gave written consent prior to participation in the study and no opposition to the publication of any type of results generated with their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
429_2022_2551_MOESM2_ESM.pdf
Supplementary file2. Axial, sagittal and coronal sections of the 23 probabilistic maps of the present AG connectivity in MNI space. For a given tract, the color bar indicates the frequency of voxels containing the tract from 50 to 100% of the participants where the tract was frequently observed (close to the total of 411 participants, actc color scale) or from 10 to 100% of the participants where the tract was less frequently observed (plasma color scale). For example, for a tract observed in all participants, a value of 80 in a voxel means that 329 out of 411 individual tracts are passing through this voxel. L left, R right. Displays made with MRIcroGL (http://www.nitrc.org/projects/mricrogl/). (PDF 11495 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petit, L., Ali, K.M., Rheault, F. et al. The structural connectivity of the human angular gyrus as revealed by microdissection and diffusion tractography. Brain Struct Funct 228, 103–120 (2023). https://doi.org/10.1007/s00429-022-02551-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02551-5