Abstract
The identification of the “paucity of transportation vesicles” and “belt-like” tight junctions (TJs) of endothelial cells as the “morphological correlate of a blood–brain barrier” (BBB) by Reese and Karnovsky (J Cell Biol 34:207–217, 1967) has become textbook knowledge, and countless studies have helped to further define the elements, functions, and dynamics of the BBB. Most work, however, has focused on parenchymal capillaries or less clearly defined “microvessels”, while a systematic study on similarities and differences between BBB architecture along the vascular tree within the brain and the meninges has been lacking. Since astrocytes induce endothelial cells to display BBB-typical characteristics by sonic hedgehog and Wnt/β-catenin signaling, we hypothesized that BBB-typical features should be most pronounced in parenchymal capillaries, where endothelium and astrocytes are separated by a basement membrane only. In contrast, this intimate contact is absent in leptomeningeal vessels, thereby potentially affecting BBB architecture. However, here, we show that claudin-3, claudin-5, zonula occludens-1, and occludin as typical constitutes of BBB TJs are comparably distributed in all segments of the parenchymal and the meningeal vascular tree of C57Bl6 mice. While electron microscopy revealed equally occluded interendothelial clefts, arterial vessels of the brain parenchyma but not within the meninges exhibited significantly longer TJ overlaps compared to capillaries. The highest density of endothelial vesicles was found in arterial vessels. Thus, endothelial expression of BBB-typical TJ proteins is not reflected by the distance to surrounding astrocytes, but electron microscopy reveals significant differences of endothelial specification along different segments of the CNS vasculature.





Similar content being viewed by others
References
Abbott NJ (2002) Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat 200:629–638
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7:41–53
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A (2011) The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334:1727–1731
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood–brain barrier. Nature 468:557–561
Arthur FE, Shivers RR, Bowman PD (1987) Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res 433:155–159
Bauer HC, Bauer H (2000) Neural induction of the blood–brain barrier: still an enigma. Cell Mol Neurobiol 20:13–28
Bauer HC, Krizbai IA, Bauer H, Traweger A (2014) “You Shall Not Pass”-tight junctions of the blood brain barrier. Front Neurosci 8:392
Bechmann I, Galea I, Perry VH (2007) What is the blood–brain barrier (not)? Trends Immunol 28:5–11
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014) The gut microbiota influences blood–brain barrier permeability in mice. Sci Transl Med 6:263–274
Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO (2003) Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 100:10623–10628
Daneman R (2012) The blood–brain barrier in health and disease. Ann Neurol 72:648–672
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA (2009) Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA 106:641–646
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468:562–566
De Bock M, Van Haver V, Vandenbroucke RE, Decrock E, Wang N, Leybaert L (2016) Into rather unexplored terrain-transcellular transport across the blood–brain barrier. Glia 64:1097–1123
del Zoppo GJ (2010) The neurovascular unit in the setting of stroke. J Intern Med 267:156–171
DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA (2001) Shear stress regulates occludin content and phosphorylation. Am J Physiol Heart Circ Physiol 281:105–113
D’Souza T, Agarwal R, Morin PJ (2005) Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem 280:26233–26240
Dyrna F, Hanske S, Krueger M, Bechmann I (2013) The blood–brain barrier. J Neuroimmune Pharmacol 8:763–773
Ehrlich P (1885) Das Sauerstoff-Bedürfnis des Organismus. Eine farbenanalytische Studie. [On the oxygen consumption of the body. A study using intravital dyes.]. Verlag von August Hirschwald, Berlin
Engelhardt B, Sorokin L (2009) The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511
Engelhardt B, Wolburg H (2004) Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol 34:2955–2963
Fischer S, Wobben M, Kleinstück J, Renz D, Schaper W (2000) Effect of astroglial cells on hypoxia-induced permeability in PBMEC cells. Am J Physiol Cell Physiol 279:935–944
Ge S, Song L, Pachter JS (2005) Where is the blood–brain barrier… really? J Neurosci Res 79:421–427
Gerhardt H, Betsholtz C (2003) Endothelial–pericyte interactions in angiogenesis. Cell Tissue Res 314:15–23
Haseloff RF, Blasig IE, Bauer HC, Bauer H (2005) In search of the astrocytic factor(s) modulating blood–brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol 25:25–39
Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ (2011) Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 199:223–229
Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO (2011) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121:431–443
Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H (1997) Induction of various blood–brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 19:13–26
Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T (2004) A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 89:503–513
Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N (1999) Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood–brain barrier. Biochem Biophys Res Commun 261:108–112
Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N (2003) Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood–brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res 290:275–288
Janzer RC, Raff MC (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325:253–257
Jiao H, Wang Z, Liu Y, Wang P, Xue Y (2011) Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood–brain barrier in a focal cerebral ischemic insult. J Mol Neurosci 44:130–139
Kaur C, Ling EA (2008) Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res 5:71–81
Kaur C, Sivakumar V, Zhang Y, Ling EA (2006) Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia 54:826–839
Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58:1–10
Lewandowski M (1900) Zur Lehre von der Cerebrospinalflüssigkeit [On the cerebrospinal fluid]. Z Klin Forsch 40:480–494
Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E (2008) Wnt/beta-catenin signaling controls development of the blood–brain barrier. J Cell Biol 183:409–417
Liu J, Jin X, Liu KJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to bloodbrain barrier damage in early ischemic stroke stage. J Neurosci 32:3044–3057
Nag S, Kapadia A, Stewart DJ (2011) Review: molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37:3–23
Nagy Z, Peters H, Hüttner I (1984) Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest 50:313–322
Neuhaus J, Risau W, Wolburg H (1991) Induction of blood–brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci 633:578–580
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood–brain barrier. Nat Med 19:1584–1596
Owens T, Bechmann I, Engelhardt B (2008) Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67:1113–1121
Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B (2011) Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol 122:601–614
Pollock H, Hutchings M, Weller RO, Zhang ET (1997) Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat 191:337–346
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Ramsauer M, Krause D, Dermietzel R (2002) Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J 16:1274–1276
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol 34:207–217
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9:267–285
Sandoval KE, Witt KA (2008) Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32:200–219
Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K (2006) Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood–brain barrier. Ultrasound Med Biol 32:1399–1409
Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM (2001) Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 153:933–946
Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T (1999) Induction of blood–brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res 35:155–164
Staehelin LA (1973) Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci 13:763–786
Stern L, Gautier R (1921) Recherches sur le liquide céphalo-rachidien. 1. Les rapports entre le liquide céphalo-rachidien et la circulation sanguine. Arch Int Physiol 17:138–192
Sun D, Lytle C, O’Donnell ME (1997) IL-6 secreted by astroglial cells regulates Na–K–Cl cotransport in brain microvessel endothelial cells. Am J Physiol 272:1829–1835
Thanabalasundaram G, Pieper C, Lischper M, Galla HJ (2010) Regulation of the blood–brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res 1347:1–10
Tsukamoto T, Nigam SK (1999) Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol 276:737–750
Utsumi H, Chiba H, Kamimura Y, Osanai M, Igarashi Y, Tobioka H, Mori M, Sawada N (2000) Expression of GFRalpha-1, receptor for GDNF, in rat brain capillary during postnatal development of the BBB. Am J Physiol Cell Physiol 279:C361–C368
Wolburg H, Lippoldt A (2002) Tight junctions of the blood–brain barrier: development, composition and regulation. Vasc Pharmacol 38:323–337
Wolburg H, Wolburg-Buchholz K, Kraus J (2003) Localization of claudin-3 in tight junctions of the blood–brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol 105:586–592
Wolburg H, Wolburg-Buchholz K, Engelhardt B (2005) Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol 109:181–190
Wong V (1997) Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol 273:1859–1867
Zhang ET, Inman CB, Weller RO (1990) Interrelationships of the pia mater and the perivascular (Virchow–Robin) spaces in the human cerebrum. J Anat 170:111–123
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201
Acknowledgments
The authors would like to thank Judith Craatz and Jana Brendler (Institute of Anatomy, University of Leipzig) for technical assistance in tissue preparation. Funding: Deutsche Forschungsgemeinschaft for funding (DFG-FOR 1336), Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
Deutsche Forschungsgemeinschaft for funding (DFG-FOR 1336), Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases).
Additional information
S. Hanske and F. Dyrna contributed equally.
I. Bechmann and M. Krueger contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2016_1267_MOESM1_ESM.tif
Supplementary Fig. 1 Expression pattern of occludin and claudin-5 was further assessed in parenchymal arteries, capillaries, and veins. Therefore, the length of individual occludin-positive TJ strands was measured and compared with the length positive for claudin-5 within the same TJ strand. Thus, a ratio of the occludin-positive TJ strand length covered by immunoreactivity for claudin-5 was used to check for differences in the expression pattern between arteries (A), capillaries (C), and veins (V). Here, differences impressively failed to reach statistical significance (p = 0.18, Kruskal–Wallis test; Dunn’s multiple comparison; n = 5; bars are given as standard deviation of the mean). Capillaries, veins, and arteries were differentiated by diameter and immunoreactivity for α-SMA. Scale bar: 10 µm; DAPI: nuclei (TIFF 4133 kb)
429_2016_1267_MOESM2_ESM.tif
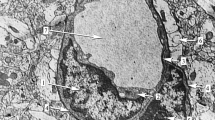
Supplementary Fig. 2 Electron micrograph illustrating the topography of perivascular spaces, here surrounding an arterial vessel. Higher magnification (right) allows clear-cut delineation of the different cellular populations and basal laminas. Of note, PVS are bordered by the outermost vascular basal lamina (highlighted in yellow) and the glial (astrocytic) basal lamina (highlighted in red). Pericytes (not shown) or smooth muscle cells (SMC) are by definition part of the vascular wall, ensheathed by the vascular basal laminas, whereas leptomeningeal mesothelial cells (arrow heads) or perivascular macrophages (not shown) are located within PVS. ‘Spaces’ around parenchymal vessels, which are not bordered by basal laminas that are often related to perfusion- or dehydration-related swelling of astrocytic endfeet, thus representing artifacts. E = endothelium, L = vascular lumen, arrow = endothelial TJ (TIFF 2305 kb)
Rights and permissions
About this article
Cite this article
Hanske, S., Dyrna, F., Bechmann, I. et al. Different segments of the cerebral vasculature reveal specific endothelial specifications, while tight junction proteins appear equally distributed. Brain Struct Funct 222, 1179–1192 (2017). https://doi.org/10.1007/s00429-016-1267-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1267-0