Abstract
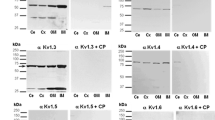
Hyperpolarization-activated cationic and cyclic nucleotide-gated channels (HCN) comprise four homologous subunits (HCN1–HCN4). HCN channels are found in excitable and non-excitable tissues in mammals. We have previously shown that HCN2 may transport ammonium (NH4 +), besides sodium (Na+), in the rat distal nephron. In the present work, we identified HCN1 and HCN3 in the proximal tubule (PT) and HCN3 in the thick ascending limb of Henle (TALH) of the rat kidney. Immunoblot assays detected HCN1 (130 kDa) and HCN3 (90 KDa) and their truncated proteins C-terminal HCN1 (93 KDa) and N-terminal HCN3 (65 KDa) in enriched plasma membranes from cortex (CX) and outer medulla (OM), as well as in brush-border membrane vesicles. Immunofluorescence assays confirmed apical localization of HCN1 and HCN3 in the PT. HCN3 was also found at the basolateral membrane of TALH. We evaluated chronic changes in mineral dietary on HCN3 protein abundance. Animals were fed with three different diets: sodium-deficient (SD) diet, potassium-deficient (KD) diet, and high-potassium (HK) diet. Up-regulation of HCN3 was observed in OM by KD and in CX and OM by HK; the opposite effect occurred with the N-terminal truncated HCN3 in CX (KD) and OM (HK). SD diet did not produce any change. Since HCN channels activate with membrane hyperpolarization, our results suggest that HCN channels may play a role in the Na+–K+-ATPase activity, contributing to Na+, K+, and acid–base homeostasis in the rat kidney.








Similar content being viewed by others
References
Alpern RJ (1985) Mechanism of basolateral membrane H+/OH−/HCO−3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol 86:613–636
Amlal H, Wang Z, Soleimani M (1998) Potassium depletion downregulates chloride-absorbing transporters in rat kidney. J Clin Investig 101:1045–1054. doi:10.1172/JCI686
Amlal H, Habo K, Soleimani M (2000) Potassium deprivation upregulates expression of renal basolateral Na(+)–HCO(3)(−) cotransporter (NBC-1). Am J Physiol Renal Physiol 279:F532–F543
Attmane-Elakeb A, Chambrey R, Tsimaratos M et al (1996) Isolation and characterization of luminal and basolateral plasma membrane vesicles from the medullary thick ascending loop of Henle. Kidney Int 50:1051–1057
Bello-Reuss E, Weber MR (1986) Electrophysiological studies on primary cultures of proximal tubule cells. Am J Physiol 251:F490–F498
Biagi BA, Sohtell M (1986) Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol 250:F267–F272
Bolívar JJ, Tapia D, Arenas G et al (2008) A hyperpolarization-activated, cyclic nucleotide-gated, (Ih-like) cationic current and HCN gene expression in renal inner medullary collecting duct cells. Am J Physiol Cell Physiol 294:C893–C906. doi:10.1152/ajpcell.00616.2006
Booth AG, Kenny AJ (1974) A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 142:575–581
Brandis M, Keyes J, Windhager EE (1972) Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222:421–427
Buerkert J, Martin D, Trigg D (1982) Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Investig 70:1–12
Buffin-Meyer B, Marsy S, Barlet-Bas C et al (1996) Regulation of renal Na+, K(+)-ATPase in rat thick ascending limb during K+ depletion: evidence for modulation of Na+ affinity. J Physiol 490(Pt 3):623–632
Calejo AI, Reverendo M, Silva VS et al (2014) Differences in the expression pattern of HCN isoforms among mammalian tissues: sources and implications. Mol Biol Rep 41:297–307. doi:10.1007/s11033-013-2862-2
Carrisoza-Gaytán R, Rangel C, Salvador C et al (2011) The hyperpolarization-activated cyclic nucleotide-gated HCN2 channel transports ammonium in the distal nephron. Kidney Int 80:832–840. doi:10.1038/ki.2011.230
Chen J, Mitcheson JS, Lin M, Sanguinetti MC (2000) Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. J Biol Chem 275:36465–36471. doi:10.1074/jbc.M007034200
Difrancesco D (1993) Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55:455–472. doi:10.1146/annurev.ph.55.030193.002323
DuBose TD, Good DW (1991) Effects of chronic hyperkalemia on renal production and proximal tubule transport of ammonium in rats. Am J Physiol 260:F680–F687
DuBose TD, Good DW (1992) Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Investig 90:1443–1449. doi:10.1172/JCI116011
Edwards RM, Pullen M, Nambi P (1999) Distribution of neutral endopeptidase activity along the rat and rabbit nephron. Pharmacology 59:45–50. doi:10.1159/000028304
Elkjaer M-L, Kwon T-H, Wang W et al (2002) Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol 283:F1376–F1388. doi:10.1152/ajprenal.00186.2002
Erdös EG, Skidgel RA (1989) Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 3:145–151
Frace AM, Maruoka F, Noma A (1992) External K+ increases Na+ conductance of the hyperpolarization-activated current in rabbit cardiac pacemaker cells. Pflugers Arch 421:97–99
Frindt G, Palmer LG (2010) Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299:F890–F897. doi:10.1152/ajprenal.00323.2010
Frömter E (1984) Viewing the kidney through microelectrodes. Am J Physiol 247:F695–F705
Garg LC, Mackie S, Tisher CC (1982) Effect of low potassium-diet on Na–K-ATPase in rat nephron segments. Pflugers Arch 394:113–117
Good DW (1996) PGE2 reverses AVP inhibition of HCO3 − absorption in rat MTAL by activation of protein kinase C. Am J Physiol 270:F978–F985
Good DW, Burg MB (1984) Ammonia production by individual segments of the rat nephron. J Clin Investig 73:602–610. doi:10.1172/JCI111250
Greger R (1985) Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 65:760–797
Gutsche HU, Peterson LN, Sauerwald KH, Levine DZ (1984) Impaired diluting capacity of the thick ascending limb during loop bicarbonate and nitrate perfusion in vivo. Can J Physiol Pharmacol 62:1416–1422
Hayashi M, Katz AI (1987) The kidney in potassium depletion. I. Na+–K+-ATPase activity and [3H] ouabain binding in MCT. Am J Physiol 252:F437–F446
Hulter HN, Toto RD, Ilnicki LP, Sebastian A (1983) Chronic hyperkalemic renal tubular acidosis induced by KCl loading. Am J Physiol 244:F255–F264
Hurtado R, Bub G, Herzlinger D (2010) The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int 77:500–508. doi:10.1038/ki.2009.483
Jaeger P, Bonjour JP, Karlmark B et al (1983) Influence of acute potassium loading on renal phosphate transport in the rat kidney. Am J Physiol 245:F601–F605
Jakobsen JK, Odgaard E, Wang W et al (2004) Functional up-regulation of basolateral Na+-dependent HCO3 − transporter NBCn1 in medullary thick ascending limb of K+-depleted rats. Pflugers Arch 448:571–578. doi:10.1007/s00424-004-1303-4
Jones JW, Sebastian A, Hulter HN et al (1982) Systemic and renal acid–base effects of chronic dietary potassium depletion in humans. Kidney Int 21:402–410
Kamm DE, Strope GL (1973) Glutamine and glutamate metabolism in renal cortex from potassium-depleted rats. Am J Physiol 224:1241–1248
Kenny AJ, Stephenson SL (1988) Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett 232:1–8
Krapf R (1988) Basolateral membrane H/OH/HCO3 transport in the rat cortical thick ascending limb. Evidence for an electrogenic Na/HCO3 cotransporter in parallel with a Na/H antiporter. J Clin Investig 82:234–241. doi:10.1172/JCI113576
Laghmani K, Preisig PA, Moe OW et al (2001) Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Investig 107:1563–1569. doi:10.1172/JCI11234
Linas SL, Peterson LN, Anderson RJ et al (1979) Mechanism of renal potassium conservation in the rat. Kidney Int 15:601–611
Ludwig A, Zong X, Jeglitsch M et al (1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393:587–591. doi:10.1038/31255
Ludwig A, Zong X, Hofmann F, Biel M (1999) Structure and function of cardiac pacemaker channels. Cell Physiol Biochem 9:179–186. doi:10.1159/000016315
Luke RG, Booker BB, Galla JH (1985) Effect of potassium depletion on chloride transport in the loop of Henle in the rat. Am J Physiol 248:F682–F687
Martinez F, Manganel M, Montrose-Rafizadeh C et al (1990) Transport of urate and p-aminohippurate in rabbit renal brush-border membranes. Am J Physiol 258:F1145–F1153
Merot J, Bidet M, Gachot B et al (1988) Patch clamp study on primary culture of isolated proximal convoluted tubules. Pflugers Arch 413:51–61
Much B, Wahl-Schott C, Zong X et al (2003) Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 278:43781–43786. doi:10.1074/jbc.M306958200
Najjar F, Zhou H, Morimoto T et al (2005) Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289:F922–F932. doi:10.1152/ajprenal.00057.2005
Nakamura S, Amlal H, Galla JH, Soleimani M (1998a) Colonic H+–K+-ATPase is induced and mediates increased HCO3 − reabsorption in inner medullary collecting duct in potassium depletion. Kidney Int 54:1233–1239. doi:10.1046/j.1523-1755.1998.00105.x
Nakamura S, Wang Z, Galla JH, Soleimani M (1998b) K+ depletion increases HCO3 − reabsorption in OMCD by activation of colonic H(+)–K(+)-ATPase. Am J Physiol 274:F687–F692
Paillard M (1998) H+ and HCO3 − transporters in the medullary thick ascending limb of the kidney: molecular mechanisms, function and regulation. Kidney Int Suppl 65:S36–S41
Pape HC (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299–327. doi:10.1146/annurev.physiol.58.1.299
Roberts KE, Magida MG, Pitts RF (1953) Relationship between potassium and bicarbonate in blood and urine. Am J Physiol 172:47–54
Silva P, Hayslett JP, Epstein FH (1973) The role of Na–K-activated adenosine triphosphatase in potassium adaptation. Stimulation of enzymatic activity by potassium loading. J Clin Investig 52:2665–2671. doi:10.1172/JCI107460
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Soleimani M, Singh G (1995) Physiologic and molecular aspects of the Na+/H+ exchangers in health and disease processes. J Investig Med 43:419–430
Stanović S, Boranić M (1998) Membrane metalloendopeptidase (CD10/CALLA): distribution, physiologic and pathophysiologic functions and its inhibitors. Lijec̆nic̆ki Vjesn 120:131–137
Stanton BA, Giebisch GH (1982) Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol 243:F487–F493
Stokke ES, Naess PA, Ostensen J et al (1993) Plasma potassium concentration as a determinant of proximal tubular NaCl and NaHCO3 reabsorption in dog kidneys. Acta Physiol Scand 148:45–54. doi:10.1111/j.1748-1716.1993.tb09530.x
Tannen RL (1970) The effect of uncomplicated potassium depletion on urine acidification. J Clin Investig 49:813–827. doi:10.1172/JCI106295
Tannen RL (1987) Effect of potassium on renal acidification and acid–base homeostasis. Semin Nephrol 7:263–273
Tannen RL, Wedell E, Moore R (1973) Renal adaptation to a high potassium intake. The role of hydrogen ion. J Clin Investig 52:2089–2101. doi:10.1172/JCI107394
Tsuruoka S, Takeda M, Yoshitomi K, Imai M (1993) Cellular heterogeneity of ammonium ion transport across the basolateral membrane of the hamster medullary thick ascending limb of Henle’s loop. J Clin Investig 92:1881–1888. doi:10.1172/JCI116780
Turner AJ, Tanzawa K (1997) Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J 11:355–364
Unwin R, Capasso G, Giebisch G (1994) Potassium and sodium transport along the loop of Henle: effects of altered dietary potassium intake. Kidney Int 46:1092–1099
Wahl-Schott C, Biel M (2009) HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66:470–494
Wainger BJ, DeGennaro M, Santoro B et al (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411:805–810. doi:10.1038/35081088
Wang Z, Baird N, Shumaker H, Soleimani M (1997) Potassium depletion and acid–base transporters in rat kidney: differential effect of hypophysectomy. Am J Physiol 272:F736–F743
Xue L, Li Y, Han X et al (2012) Investigation of hyperpolarization-activated cyclic nucleotide-gated channels in interstitial cells of Cajal of human bladder. Urology 80(224):e13–e18. doi:10.1016/j.urology.2012.04.005
Ye B, Nerbonne JM (2009) Proteolytic processing of HCN2 and co-assembly with HCN4 in the generation of cardiac pacemaker channels. J Biol Chem 284:25553–25559. doi:10.1074/jbc.M109.007583
Zha Q, Brewster AL, Richichi C et al (2008) Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: role of N-linked glycosylation. J Neurochem 105:68–77. doi:10.1111/j.1471-4159.2007.05110.x
Zong X, Stieber J, Ludwig A et al (2000) A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J Biol Chem 276:6313–6319. doi:10.1074/jbc.M010326200
Acknowledgments
We thank Rolando Carrisoza Gaytan, Maria Jose Gomora (confocal microscope, Macroproyecto SDI-PTID.05.01), Lucia Macias Rosales and Hector Rico Morales for their technical assistance; Lisa Satlin for reading an early draft of this manuscript and PhD program Ciencias Biologicas at the Universidad Nacional Autonoma de Mexico (UNAM). This work was supported by grants PAPIIT IN214613 (UNAM), Conacyt 166913 and scholarships from Conacyt (Zinaeli Lopez-Gonzalez, 175629) and PAPIIT IN214613 (Cosete Ayala-Aguilera, Zinaeli Lopez Gonzalez).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared no competing interests.
Rights and permissions
About this article
Cite this article
López-González, Z., Ayala-Aguilera, C., Martinez-Morales, F. et al. Immunolocalization of hyperpolarization-activated cationic HCN1 and HCN3 channels in the rat nephron: regulation of HCN3 by potassium diets. Histochem Cell Biol 145, 25–40 (2016). https://doi.org/10.1007/s00418-015-1375-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-015-1375-6