Abstract
Background
A noticeable change of the male-to-female sex ratio (SR) has been observed in Amyotrophic Lateral Sclerosis (ALS) leading to an apparent regression of SR with time (SR close to 1:1).
Objective
To provide a global SR estimate and investigate its relation with respect to population age.
Methods
A systematic review and meta-analysis was conducted including only population-based studies with a high-quality methodology in European ancestral origin population. Male-to-female SR was estimated by three different measures: SR number, SR crude incidence and SR standardized incidence. Standard and dose–response meta-analyses were performed to assess the pooled SR measures (irrespective of population age) and the evolution of the SR measures with respect to population age, respectively. Potential sources of heterogeneity were investigated via meta-regression.
Results
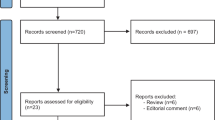
Overall, 3254 articles were retrieved in the literature search. Thirty-nine studies stratified by time periods were included. The overall pooled male-to-female ratio was 1.28 (95% CI 1.23–1.32) for SR number, 1.33 (95% CI 1.29–1.38) for SR crude incidence and 1.35 (95% CI 1.31–1.40) for SR standardized incidence. The SR number with respect to population age reveals a progressive reduction of SR at increasing age, while the SR crude incidence in relation to age displays a U-shaped curve.
Conclusions
The number and the incidence of ALS cases were consistently higher in males than females. Dose–response meta-analysis showed that SR measures change with respect to population age. Further original research is needed to clarify if our findings are reproducible in other populations.




Similar content being viewed by others
Data availability
The data for the analyses described in this paper are available by request from the authors.
References
Beghi E, Logroscino G, Chiò A et al (2006) The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta 1762:1150–1157. https://doi.org/10.1016/j.bbadis.2006.09.008
Logroscino G, Traynor BJ, Hardiman O et al (2008) Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 79:6–11. https://doi.org/10.1136/jnnp.2006.104828
Luna J, Logroscino G, Couratier P, Marin B (2017) Current issues in ALS epidemiology: variation of ALS occurrence between populations and physical activity as a risk factor. Rev Neurol (Paris) 173:244–253. https://doi.org/10.1016/j.neurol.2017.03.035
Chiò A, Mora G, Moglia C et al (2017) Secular trends of amyotrophic lateral sclerosis: the piemonte and valle d’Aosta register. JAMA Neurol 74:1097–1104. https://doi.org/10.1001/jamaneurol.2017.1387
Marin B, Boumédiene F, Logroscino G et al (2017) Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol 46:57–74. https://doi.org/10.1093/ije/dyw061
Marin B, Logroscino G, Boumédiene F et al (2016) Clinical and demographic factors and outcome of amyotrophic lateral sclerosis in relation to population ancestral origin. Eur J Epidemiol 31:229–245. https://doi.org/10.1007/s10654-015-0090-x
Marin B, Fontana A, Arcuti S et al (2018) Age-specific ALS incidence: a dose-response meta-analysis. Eur J Epidemiol 33:621–634. https://doi.org/10.1007/s10654-018-0392-x
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012. https://doi.org/10.1001/jama.283.15.2008
Fiest KM, Pringsheim T, Patten SB et al (2014) The role of systematic reviews and meta-analyses of incidence and prevalence studies in neuroepidemiology. Neuroepidemiology 42:16–24. https://doi.org/10.1159/000355533
Mitsumoto H, Chad DA, Pioro EP (1998) Amyotrophic lateral sclerosis. Oxford University Press, Oxford
Szklo M (1998) Population-based cohort studies. Epidemiol Rev 20:81–90. https://doi.org/10.1093/oxfordjournals.epirev.a017974
(2000) Census, race and science. Nat Genet 24:97–98. https://doi.org/10.1038/72884
United Nations Statistics Division Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. https://unstats.un.org/unsd/methodology/m49/
Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ (2002) Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology 59:280–282. https://doi.org/10.1212/wnl.59.2.280
United Nations Demographic yearbook. DYB annual issues. https://unstats.un.org/unsd/demographic-social/products/dyb/index.cshtml
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30. https://doi.org/10.1093/oxfordjournals.epirev.a036298
van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21:589–624. https://doi.org/10.1002/sim.1040
Higgins J, Thomas J (2011) Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration
Manjaly ZR, Scott KM, Abhinav K et al (2010) The sex ratio in amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler Off Publ World Fed Neurol Res Group Mot Neuron Dis 11:439–442. https://doi.org/10.3109/17482961003610853
Trojsi F, D’Alvano G, Bonavita S, Tedeschi G (2020) Genetics and sex in the pathogenesis of amyotrophic lateral sclerosis (ALS): is there a link? Int J Mol Sci. https://doi.org/10.3390/ijms21103647
Deeks JJ, Higgins JP, Douglas DG, on behalf of the Cochrane Statistical Methods Group (2019) Chapter 10: analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions, 2nd Edition. John Wiley & Sons, Chichester (UK)
Radhakrishnan K, Ashok PP, Sridharan R, Mousa ME (1986) Descriptive epidemiology of motor neuron disease in Benghazi, Libya. Neuroepidemiology 5:47–54. https://doi.org/10.1159/000110812
Sajjadi M, Etemadifar M, Nemati A et al (2010) Epidemiology of amyotrophic lateral sclerosis in Isfahan. Iran Eur J Neurol 17:984–989. https://doi.org/10.1111/j.1468-1331.2010.02972.x
Ramanathan RS, Rana S (2018) Demographics and clinical characteristics of primary lateral sclerosis: case series and a review of literature. Neurodegener Dis Manag 8:17–23. https://doi.org/10.2217/nmt-2017-0051
Wijesekera LC, Mathers S, Talman P et al (2009) Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology 72:1087–1094. https://doi.org/10.1212/01.wnl.0000345041.83406.a2
Logroscino G, Marin B, Piccininni M et al (2018) Referral bias in ALS epidemiological studies. PLoS ONE 13:e0195821. https://doi.org/10.1371/journal.pone.0195821
Finegan E, Chipika RH, Li Hi Shing S et al (2019) The clinical and radiological profile of primary lateral sclerosis: a population-based study. J Neurol 266:2718–2733. https://doi.org/10.1007/s00415-019-09473-z
McCombe PA, Henderson RD (2010) Effects of gender in amyotrophic lateral sclerosis. Gend Med 7:557–570. https://doi.org/10.1016/j.genm.2010.11.010
Alonso A, Logroscino G, Jick SS, Hernán MA (2010) Association of smoking with amyotrophic lateral sclerosis risk and survival in men and women: a prospective study. BMC Neurol 10:6. https://doi.org/10.1186/1471-2377-10-6
Weisskopf MG, McCullough ML, Calle EE et al (2004) Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol 160:26–33. https://doi.org/10.1093/aje/kwh179
Chiò A, Moglia C, Canosa A et al (2020) ALS phenotype is influenced by age, sex, and genetics: a population-based study. Neurology 94:e802–e810. https://doi.org/10.1212/WNL.0000000000008869
Acknowledgements
We thank the following main authors or co-authors of population-based articles who answered our solicitation and for the useful material that they were able to provide: Kari Murros, Poul Joensen, Raeburn Forbes, Robert Swingler (the Scottish MND Register is funded by MND Scotland and supported by the Anne Rowling Regenerative Neurology Clinic), Ibrahim Imam, James Rooney, Albert Ludolph, Gabriele Nagel, Marwa Elamin, Ammar Al-Chalabi, Orla Hardiman, Mark Heverin, Mark Huisman, Joachim Wolf, Adriano Chio, Federica Pisa, Jessica Mandrioli, Monica Bandettini, Stefano Zocollela, Maura Pugliatti, Leslie Parish, Paolo Ragonese, Valerie Mc Guire, Will Longstreth, Eric J. Sorenson, Farrah Mateen, James D. Bonaparte, Cristina Vazquez, Carlos Ketzoian, Kurupath Radhakrishnan, Chien-Hsu Lai, Chung Yan G Fong, Hitoshi Okumura, Tameko Kihira, Bruce Taylor and A Lannuzel. We thank Paul Mehta, Heather Jordan and Jhaqueline Valle for providing data to calculate US incidence. The data came from surveillance projects funded by the Agency for Toxic Substances and Disease Registry’s (ATSDR) National ALS Registry [www.cdc.gov/als] (Contract #200-2009- 32577 and Contract #200-2010-F-36614). We also thank Walter Rocca and Brandon R. Grossardt for the detailed data on Olmsted county population with which they provided us, and Hidenao Sasaki, Robert Miller and Eric Denys as contact persons. We thank Vanna Pistotti for her advice during the literature search, as well as Mineko Terao, Lorenzo Moja and Claudio Pelucchi. We thank Limoges teaching hospital for its grant initiative for mobility.
Funding
This research received no specific Grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this paper. Conceptualization: AF, BM, MC; data curation: AF, BM; statistical analysis: AF, BM; interpretation; AF, BM, JL; writing-original draft: AF, BM, JL; all authors reviewed the first draft. All authors contributed to the final manuscript in terms of intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
Andrea Fontana, Benoit Marin, Jaime Luna, Giancarlo Logroscino, Farid Boumédiene, Pierre-Marie Preux, Philippe Couratier and Massimilano Copetti declare no disclosures relevant to the manuscript. Ettore Beghi declares to collaborate with the Italian Ministry of Health, SOBI, Arvelle Therapeutics and the American ALS Association.
Ethical approval
As this review of the literature/meta-analysis does not involve ALS patients but makes use of publications concerning ALS, informed consent of patients is not applicable. Not even approval of an ethics committee is applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fontana, A., Marin, B., Luna, J. et al. Time-trend evolution and determinants of sex ratio in Amyotrophic Lateral Sclerosis: a dose–response meta-analysis. J Neurol 268, 2973–2984 (2021). https://doi.org/10.1007/s00415-021-10464-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10464-2