Abstract
Background
Previous epidemiologic studies have examined the association of smoking with amyotrophic lateral sclerosis (ALS) incidence, but their results have been inconsistent. Moreover, limited information exists on the association between smoking and survival in ALS patients. We evaluated the association of smoking with ALS incidence and survival in a population-based cohort.
Methods
We conducted a case-control study nested in the General Practice Research Database, a computerized clinical database in the United Kingdom. Cases were 1143 individuals with a diagnosis of ALS; 11,371 matched controls were selected among GPRD participants free of ALS. Predictors of survival were determined in the ALS cases. Smoking information was obtained from the computer database.
Results
Smoking was not associated with the risk of ALS in this population. The rate ratio (RR) of ALS comparing ever versus never smokers was 1.04, 95% confidence interval (CI) 0.80-1.34. In analysis stratified by gender, however, ever smoking was associated with ALS in women (RR 1.53, 95% CI 1.04-2.23) but not in men (RR 0.75, 95% CI 0.53-1.06). Mortality was 71% after 2.1 average years of follow-up. Old age and female sex were associated with lower survival. Smoking was a predictor of mortality only in women. Comparing ever versus never smokers, RR (95% CI) of death was 1.31 (1.04-1.65) in women, and 0.90 (0.72-1.11) in men.
Conclusion
In this large population-based study, smoking was associated with ALS risk and worse survival in women but not in men.
Similar content being viewed by others
Background
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the progressive loss of upper and lower motor neurons. Although its etiology remains unknown, certain environmental (non-genetic) factors have been postulated as potential causative factors[1]. Cigarette smoking, in particular, has attracted interest as a risk factor for ALS. Smoking could increase the risk of ALS through several mechanisms, including inflammation, oxidative stress, and neurotoxicity caused by heavy metals and other chemical compounds present in cigarette smoke[2–5].
A number of epidemiologic studies have assessed the association of cigarette smoking with ALS incidence, but their results have been inconsistent[6]. More recently, a systematic review and meta-analysis has suggested that sex could modify the association between smoking and ALS risk, with smoking linked to higher risk of ALS in women but not in men[7]. Similarly, the only three studies that estimated the association of smoking with the survival of ALS patients were inconclusive[8–10].
Here we present estimates of the association of smoking with ALS risk, and with survival in ALS patients, in the General Practice Research Database (GPRD), a large clinical database in the United Kingdom (UK).
Methods
Study population
The GPRD has been described in detail elsewhere[11, 12]. Briefly, starting in 1987, selected general practitioners in the UK agreed to record electronically their patients' clinical information and provide these anonymized data for research purposes. Recorded information includes demographic variables, symptoms, lifestyles, such as smoking, clinical variables, such as body mass index, medical diagnoses, drug prescriptions, referrals to and diagnosis from specialists, and hospital admissions. Medical records, including hospital discharge and referral letters, are available for review upon request. Since its inception, more than 5 million UK residents have been included in the database. The GPRD population is representative of the British population with regard to age, gender, and geographic distribution[12]. Additionally, different validation studies have shown that data contained in the GPRD is of enough quality and completeness to be used for epidemiologic analysis[13–16].
The GPRD is managed by the Medicines and Healthcare Products Regulatory Agency in the UK. The present study has been approved by the Institutional Review Board at the University of Minnesota.
ALS ascertainment
Cases of ALS were identified from the population of patients older than age 20 enrolled in the GPRD for at least two years from January 1990 to July 2008. Any participant with a computer diagnosis of motor neuron disease, ALS, progressive muscular atrophy, progressive bulbar palsy, or primary lateral sclerosis was considered a case. In a review of medical charts and death certificates of 65 potential ALS cases identified following this approach, we were able to confirm 85% of them[17]. Similarly, incidence rates of computer-diagnosed ALS in the GPRD were comparable to those found in population-based registries in Scotland and Ireland[17].
Study design
To estimate the association of smoking with ALS risk, we conducted a nested case-control study. Cases were individuals with ALS with at least two years of follow-up before the date of diagnosis. Up to 10 controls free of ALS were selected for each case matched by age (± 3 years), gender, practice, and year of enrolment in the GPRD. Controls had to be alive and free of ALS at the date of diagnosis of their corresponding case (index date).
The association of smoking with ALS survival was assessed using a cohort design. Follow-up started on the date of diagnosis and continued until the patient died, transferred out to a different practice, or the end of July 2008, whichever occurred earlier.
Exposure assessment
Information on smoking status before the index date was obtained from the computer database using the earliest smoking information for each study participant. Individuals were classified as never, past, or current smokers, or as having missing information. Detailed information on amount of smoking was not available but, as a surrogate marker of smoking intensity, we collected information on smoking cessation advice or treatment, and whether participants were labeled as 'heavy smokers'. In a previous publication, smokers receiving advice or treatment had a higher risk of lung cancer than other smokers, supporting the validity of this classification[18]. If a smoker in our population received smoking cessation advice or treatment, or was labeled as 'heavy smoker', we considered him or her to be a heavy smoker, and a non-heavy smoker otherwise. In the sample of controls, heavy smokers had a higher mortality than non-heavy smokers, and the non-heavy smokers had a higher mortality than never smokers, supporting the validity of our smoking classification: in an analysis adjusted for age and sex, the mortality rate ratio (RR) was 2.19 (95% confidence interval [CI] 1.71-2.80) in heavy smokers and 1.17 (95% CI 1.05-1.30) in non-heavy smokers compared to never smokers. Additionally, we obtained information on body mass index from the database. Information on smoking was missing in 18% of the study sample (19% in men and 17% in women).
Statistical analysis
In the case-control study, we estimated the RR of ALS for smoking status, with never smokers as the reference group, via conditional logistic regression models adjusted for matching factors. The main analysis included all cases and their respective controls. We performed an additional analysis including only individuals with at least 5 years of recorded medical information before their index date. For this analysis, we advanced the index date by 5 years and only used smoking information recorded before this earlier date.
In the cohort study we estimated the mortality RR for smoking status via a Cox proportional hazards model with days from diagnosis to end of follow-up, as the time variable. In addition to smoking status, we included in the model the following variables: age at diagnosis, gender, diagnostic classification (ALS/motor neuron disease vs. ALS variants), and calendar year. Information on site of ALS onset was not available. Calendar year was modeled fitting a restricted cubic spline function with 3 knots. We excluded 12 cases in which date of diagnosis was the same as date of death. An additional analysis was conducted excluding individuals diagnosed with ALS variants. Adjusted survival was estimated in four groups defined by gender and smoking status (ever, never) averaging survival curves for each individual in the sample, using a SAS macro developed by Zhang et al.[19]
In both the case-control and cohort analyses, we estimated separate RR in men and women, and assessed their heterogeneity using the likelihood ratio test. Finally, we repeated all analyses including only individuals younger than 75 (800 cases, 8114 controls), since ALS diagnosis in older individuals is less certain.
Results
During the study period, we identified 1143 ALS cases with at least two years of follow-up before their diagnosis, and selected 11,371 matched controls. Characteristics of cases and controls are presented in table 1. The median age in both groups was 67.
The association of smoking with ALS risk is presented in table 2. Compared with never smokers, the RR (95% CI) of ALS for ever smokers was 1.04 (0.80-1.34). The corresponding RR (95% CI) was 1.53 (1.04-2.23) in women and 0.75 (0.53-1.06) in men. This gender difference was present in young and older individuals. In those older than 50, the RR (95% CI) of ALS for ever smokers compared with never smokers was 0.74 (0.51-1.07) in men and 1.49 (1.00-2.22) in women. In those 50 and younger, the RR (95% CI) were 0.84 (0.33-2.19) in men and 2.14 (0.51-9.04) in women.
Results were similar when we included only cases and controls with at least five years of follow-up before their index date (table 2), when we adjusted for body mass index (data not shown), and when we excluded individuals older than 75 on the index date: the RR (95% CI) was 0.77 (0.52-1.15) in men and 1.50 (0.96-2.35) in women. The RRs of ALS for heavy smokers and non-heavy smokers were of similar magnitude compared with never smokers. The RR (95% CI) of ALS in heavy smokers compared with never smokers was 1.08 (0.74-1.56) in the entire cohort, 0.72 (0.44-1.19) in men and 1.77 (1.02-3.09) in women. The corresponding figures comparing non heavy smokers with never smokers were 1.03 (0.80-1.33), 0.75 (0.53-1.06) and 1.50 (1.02-2.21).
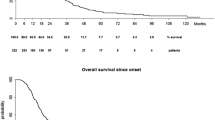
Of 1131 ALS cases with at least one day of follow-up after their diagnosis, 802 (70.9%) died, 108 (9.6%) transferred out, and 221 (19.5%) were alive at the time of last recording in the GPRD, after an average follow-up of 2.1 years. Median survival after diagnosis was 1.5 years. Old age and female gender were strongly associated with mortality. Each 5-year increment in age was associated with 20% higher mortality rate (RR 1.20, 95% CI, 1.16-1.24). Similarly, women had a 22% higher mortality than men (RR 1.22, 95% CI, 1.06-1.41). Compared with never smokers, the mortality RR (95% CI) was 1.45 (1.03-2.03) for heavy smokers and close to 1 for all other groups (table 3). Compared with never smokers, the mortality RR (95% CI) for ever smokers was 1.31 (1.04-1.65) in women and 0.90 (0.72-1.11) in men. The risk was particularly elevated in female heavy smokers (HR 1.94, 95% CI 1.24-3.06). As shown in figure 1, adjusted survival was lower in women who were ever smokers compared to men and women never smokers. Results were similar when we excluded individuals diagnosed with an ALS variant.
Discussion
In this large prospective study, smoking was a predictor or ALS in women, and of mortality in female ALS patients, but not in men.
Smoking has been associated with several neurodegenerative disorders. Prospective investigations have found a higher risk of dementia and a lower risk of Parkinson's disease in smokers compared to non-smokers[20, 21]. The epidemiologic evidence regarding smoking and ALS is less consistent, with some studies showing a higher risk of ALS among smokers,[22–24] and others not finding any clear association[25]. Notably, in the Cancer Prevention Study II cohort, a prospective cohort including over 1 million people, results were similar to ours: ever smoking was associated with a higher risk of ALS mortality in women (HR 1.32, 95% CI 1.03-1.69) but with a lower risk in men (HR 0.77, 95% CI 0.59-1.00)[26]. Consistent with this observation, a recent systematic review and meta-analysis showed that the proportion of men and women in a particular study made a major contribution to between-study heterogeneity[7]. The estimated pooled RR of ALS in ever versus never smokers was 0.87 (95% CI 0.71-1.06) in men, and 1.58 (95% CI 1.21-2.08) in women[7]. Results from the GPRD are in agreement with the previous meta-analysis and reinforce its conclusions.
Smoking could increase the risk of ALS through different mechanisms. Strong evidence supports the role of smoking as a cause of oxidative damage,[2] and oxidative damage has been involved in the pathogenesis of ALS[27]. Also, more than 4 thousand different compounds have been identified in tobacco and cigarette smoke, including heavy metals, such as lead or cadmium, and formaldehyde[28]. Although the role of these in ALS pathogenesis has not been defined, some studies point to an increased risk of ALS and worse ALS survival associated with higher levels of lead exposure,[8, 29] and to a potential higher incidence of ALS in individuals exposed to formaldehyde[30]. Systemic inflammation, another well-known effect of smoking, might also be involved[4]. Biological mechanisms supporting the difference in the association of smoking with ALS in the two genders are less clear, though prior observations suggest that women could be more sensitive to deleterious effects of cigarette smoking, probably through differences in the metabolism of tobacco compounds[31, 32]. Additional evidence suggests that the association of smoking with different health outcomes, including lung disease or thyroid disorders, might differ by gender[32, 33].
More intriguing is the sex difference in the association of smoking with survival. Only three previous studies have reported associations of smoking with ALS prognosis. Kamel et al studied the association of different exposures, including smoking, with survival in a group of 100 ALS cases in New England. In age and sex-adjusted analysis, ever smokers had a better survival than never smokers (HR 0.6, 95% CI 0.4-1.0, with time from diagnosis to death as the outcome, or HR 0.7, 95% CI 0.5-1.2, with time from onset of symptoms to death as outcome)[8]. In a report including 180 ALS patients from Washington State, smoking did not predict mortality: compared to never smokers, the HR of death in current smokers was 1.1 (95% 0.7-1.6) and 1.0 (95% CI 0.7-1.4) in past smokers[9]. Neither study assessed the association separately in men and women. Finally, smoking was not a significant predictor of survival in a group of 2069 ALS patients receiving riluzole in France, but the actual estimates of association were not reported[10].
Strengths of our study include the large sample size, providing enough statistical power for subgroup analysis, and the selection of cases and controls from a well-defined cohort, reducing the possibility of selection bias. Several limitations, however, might reduce the validity of our results. First, we have not confirmed all ALS cases identified in the GPRD, though a previous study suggested acceptable validity of the computer diagnosis[17]. Because validity of ALS diagnosis could be lower in older individuals,[34] we repeated analyses after excluding those older than 75, obtaining similar results. Second, smoking status was classified in broad categories (never, non-heavy smokers, heavy smokers, unknown). Thus, we were not able to determine precise dose-response relationships. Lastly, unmeasured confounding could explain our results if a risk factor for ALS was associated with smoking and distributed unevenly in men and women.
Conclusions
We have found that smoking is associated with higher risk of ALS and worse survival in women but not in men. Future studies should focus on exploring the mechanisms that might explain the observed associations.
References
Armon C: Amyotrophic lateral sclerosis. Neuroepidemiology From principles to practice. Edited by: Nelson LM, Tanner CM, Van Den Eeden SK, McGuire VM. 2004, New York: Oxford University Press, 162-187.
Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ: Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers -- smoking as a cause of oxidative damage. N Engl J Med. 1995, 332 (18): 1198-1203. 10.1056/NEJM199505043321804.
Chiba M, Masironi R: Toxic and trace elements in tobacco and tobacco smoke. Bulletin of the World Health Organization. 1992, 70 (2): 269-275.
Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM: Systemic effects of smoking. Chest. 2007, 131 (5): 1557-1566. 10.1378/chest.06-2179.
Weisskopf MG, Ascherio A: Cigarettes and amyotrophic lateral sclerosis: only smoke or also fire?. Ann Neurol. 2009, 65 (4): 361-362. 10.1002/ana.21700.
Armon C: An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003, 22: 217-228. 10.1159/000070562.
Alonso A, Logroscino G, Hernán MA: Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatr. 2010, 163 (11): 1021-30.
Kamel F, Umbach DM, Stallone L, Richards M, Hu H, Sandler DP: Association of lead exposure with survival in amyotrophic lateral sclerosis. Environ Health Perspect. 2008, 116: 943-947. 10.1289/ehp.11193.
del Aguila MA, Longstreth WT, McGuire V, Koepsell TD, van Belle G: Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003, 60: 813-819. 10.1001/archneur.60.6.813.
Paillisse C, Lacomblez L, Dib M, Bensimon G, Garcia-Acosta S, Meininger V: Prognostic factors for survival in amyotrophic lateral sclerosis patients treated with riluzole. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005, 6: 37-44. 10.1080/14660820510027035.
Wood L, Martinez C: The General Practice Research Database. Role in pharmacovigilance. Drug Safety. 2004, 27 (12): 871-881. 10.2165/00002018-200427120-00004.
Lawson DH, Sherman V, Hollowell J: The General Practice Research Database. Scientific and Ethical Advisory Group. QJM. 1998, 91 (6): 445-452. 10.1093/qjmed/91.6.445.
Jick H, Jick SS, Derby LE: Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991, 302: 766-768. 10.1136/bmj.302.6779.766.
Jick H, Terris BZ, Derby LE, Jick SS: Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiol Drug Saf. 1992, 1: 347-349. 10.1002/pds.2630010607.
Jick SS, Kaye JA, Vasilakis-Scaramozza C, García Rodríguez LA, Ruigomez A, Meier CB, Schlienger RG, Black C, Jick H: Validity of the General Practice Research Database. Pharmacotherapy. 2003, 23 (5): 686-689. 10.1592/phco.23.5.686.32205.
García Rodríguez LA, Pérez Gutthann S: Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998, 45 (5): 419-425. 10.1046/j.1365-2125.1998.00701.x.
Alonso A, Logroscino G, Jick SS, Hernán MA: Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur J Neurol. 2009, 16: 745-751. 10.1111/j.1468-1331.2009.02586.x.
Hernández-Díaz S, García Rodríguez LA: Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer. 2007, 120: 1565-1572. 10.1002/ijc.22514.
Zhang X, Loberiza FR, Klein JP, Zhang M-J: A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007, 88: 95-101. 10.1016/j.cmpb.2007.07.010.
Hernán MA, Alonso A, Logroscino G: Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008, 19 (3): 448-450. 10.1097/EDE.0b013e31816bbe14.
Hernán MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ: A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002, 52 (3): 276-284. 10.1002/ana.10277.
Kamel F, Umbach DM, Munsat TL, Shefner JM, Sandler DP: Association of cigarette smoking with amyotrophic lateral sclerosis. Neuroepidemiology. 1999, 18: 194-202. 10.1159/000026211.
Nelson LM, McGuire V, Longstreth WT, Matkin C: Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol. 2000, 151 (2): 156-163.
Gallo V, Bueno-De-Mesquita HB, Vermeulen R, Andersen PM, Kyrozis A, Linseisen J, Kaaks R, Allen NE, Roddam AW, et al: Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol. 2009, 65: 378-385. 10.1002/ana.21653.
Fang F, Bellocco R, Hernán MA, Ye W: Smoking, snuff dipping and the risk of amyotrophic lateral sclerosis - a prospective cohort study. Neuroepidemiology. 2006, 27 (4): 217-221. 10.1159/000096956.
Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A: Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004, 160 (1): 26-33. 10.1093/aje/kwh179.
Rothstein JD: Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009, 65 (suppl): S3-S9. 10.1002/ana.21543.
Hoffmann D, Hoffmann I, El-Bayoumy K: The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001, 14 (7): 767-790. 10.1021/tx000260u.
Kamel F, Umbach DM, Hu H, Munsat TL, Shefner JM, Taylor JA, Sandler DP: Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener Dis. 2005, 2 (3-4): 195-201. 10.1159/000089625.
Weisskopf MG, Morozova N, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Ascherio A: Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2009, 80 (5): 558-561. 10.1136/jnnp.2008.156976.
Gan W, Man SF, Postma D, Camp P, Sin D: Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006, 7 (1): 52-10.1186/1465-9921-7-52.
Sin DD, Cohen SB-Z, Day A, Coxson H, Paré PD: Understanding the biological differences in susceptibility to chronic obstructive pulmonary disease between men and women. Proc Am Thorac Soc. 2007, 4: 671-674. 10.1513/pats.200706-082SD.
Vestergaard P: Smoking and thyroid disorders - a meta-analysis. Eur J Endocrinol. 2002, 146: 153-161. 10.1530/eje.0.1460153.
Chio A, Ciccone G, Calvo A, Vercellino M, Di Vito N, Ghiglione P, Mutani R, the Piemonte and Valle d'Aosta Register for ALS: Validity of hospital morbidity records for amyotrophic lateral sclerosis. A population-based study. J Clin Epidemiol. 2002, 55: 723-727. 10.1016/S0895-4356(02)00409-2.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/10/6/prepub
Acknowledgements
Alvaro Alonso had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was partially supported by a Grant-in-Aid from the Graduate School, University of Minnesota to Alvaro Alonso and grant NIH/NIA P30 AG024409-03 from the Program on the Global Epidemiology of Aging at Harvard University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AA performed statistical analysis, obtained funding and prepared the first draft of the manuscript. GL participated in study design and the interpretation of results. SSJ obtained the data and contributed to the interpretation of results. MAH participated in study design and interpretation of results, and obtained funding. All authors reviewed critically the manuscript for important intellectual content and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Alonso, A., Logroscino, G., Jick, S.S. et al. Association of smoking with amyotrophic lateral sclerosis risk and survival in men and women: a prospective study. BMC Neurol 10, 6 (2010). https://doi.org/10.1186/1471-2377-10-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-10-6