Abstract
Introduction
The estimation of post-mortem interval (PMI) remains a major challenge in forensic science. Most of the proposed approaches lack the reliability required to meet the rigorous forensic standards.
Objectives
We applied 1H NMR metabolomics to estimate PMI on ovine vitreous humour comparing the results with the actual scientific gold standard, namely vitreous potassium concentrations.
Methods
Vitreous humour samples were collected in a time frame ranging from 6 to 86 h after death. Experiments were performed by using 1H NMR metabolomics and ion capillary analysis. Data were submitted to multivariate statistical data analysis.
Results
A multivariate calibration model was built to estimate PMI based on 47 vitreous humour samples. The model was validated with an independent test set of 24 samples, obtaining a prediction error on the entire range of 6.9 h for PMI < 24 h, 7.4 h for PMI between 24 and 48 h, and 10.3 h for PMI > 48 h. Time-related modifications of the 1H NMR vitreous metabolomic profile could predict PMI better than potassium up to 48 h after death, whilst a combination of the two is better than the single approach for higher PMI estimation.
Conclusion
The present study, although in a proof-of-concept animal model, shows that vitreous metabolomics can be a powerful tool to predict PMI providing a more accurate estimation compared to the widely studied approach based on vitreous potassium concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining the post-mortem interval (PMI) has always been a major challenge for forensic pathologists. Although different methods have been developed over the years [1,2,3,4,5,6,7,8,9,10], an accurate PMI estimation, highly required in the forensic setting, is still difficult to be obtained.
In consideration of its peculiar anatomy and physiology, the eye has thoroughly been studied for forensic purposes [11,12,13,14]. In particular, vitreous humour (VH) has been a biofluid of choice for forensic purposes [15], being investigated through chemical, biochemical, toxicological, and metabolomic approaches to address not only the cause but also the time since death. The post-mortem modifications in VH potassium concentration [K+] have probably been the most studied biological parameter to infer the time since death [15].
Recently, multiparametric approaches, such as -omics sciences, appear more suitable to study the multitude of biological phenomena occurring in the post-mortem compared to the traditional methods based on single or few parameters [16,17,18,19,20]. Amongst them, metabolomics, gradually taking over in forensic research [21, 22], appears suitable to intercept time-related modifications in the early-mid PMI window (up to 72/96 h). In fact, a growing body of evidence suggests that elapsing time represents the main factor driving metabolome modifications in the post-mortem [23]. Some of the authors have already demonstrated the feasibility of VH metabolome analysis with 1H NMR approach [24].
Recently, we applied 1H NMR metabolomics to study post-mortem modifications of aqueous humour in an animal model (Ovis aries) for PMI estimation [25] using potassium analysis for comparison with an established approach [26]. Through a rigorous statistical evaluation, it was possible to estimate PMI with a prediction error of 100 min on the entire time range investigated (24 h). The results suggested a shared biological phenomenon driving the modifications of both metabolome and [K+], being the former able to explain most of the information carried by potassium but showing a greater predictive power in PMI estimation.
The paramount need to investigate higher PMIs fostered the translation of this approach to the VH. To the best of the authors’ knowledge, this is the first work in which VH metabolome and potassium are investigated and compared for an accurate estimation of PMI in a controlled animal model.
Materials and methods
Sample collection and preparation
Thirty-six heads from young adult female sheep (Ovis aries) belonging to the same herd were obtained from a local slaughterhouse after animal sacrifice (for meat consumption) by incision of the jugular vein after electrical stunning. Sheep heads are discarded as waste material, and, consequently, formal approval from the local Ethic Committee was not required for the experimental procedure. All animals were aged between 24 and 48 months and had passed standard controls for food consumption. Sheep heads were rapidly transported to the morgue of the Forensic Science Unit of the University of Cagliari and kept under controlled conditions of humidity (50 ± 5%) and temperature (25 ± 2 °C) for all the period of VH collection. Sampling was started at 6 h after death to allow adequate VH collection for metabolomics, since it was shown that in this time window VH metabolomic composition may be affected by topographical differences of VH sampling [27]. Approximately 1 mL of VH was collected from intact heads using a 5-mL G22 syringe through a single scleral puncture in the lateral canthus. Sampling was carried out at different post-mortem intervals, ranging from 6 to 84 h. Any eye was sampled once only, to avoid possible contamination. The VH samples taken from the two eyes of the same animal were collected at different times after death. After collection, the VH samples were centrifuged at 13,000 g for 5 min to remove any solid debris, mixed with 10 μL of an aqueous solution of sodium azide (NaN3, 10% w/w) in order to prevent bacterial growth, and immediately frozen at − 80 °C. A stratified random selection procedure based on the different ranges of PMI was applied to select 47 VH samples for the training set. The remaining 24 samples were used for the test set. One sample was excluded due to blood contamination.
VH sample preparation for NMR analysis
Before NMR analysis, samples were thawed and ultrafiltered using a 30-kDa filter unit (Amicon-30kDa; Merck Millipore, Darmstadt, Germany) for 10 min at 13,000 g and 4 °C in order to remove macromolecules and active enzymes. Filters were previously washed out from glycerol by adding 500 μL of distilled water and by centrifuging for 10 min at 10,000 rpm at room temperature for 15 times. For the NMR analysis, 250 μL of filtered VH was diluted with 350 μL of a 0.33-M phosphate buffer solution (pH=7.4) in D2O (99.9%, Cambridge Isotope Laboratories Inc., Andover, USA) containing the internal standard sodium 3 (trimethylsilyl)propionate-2,2,3,3,-d4 (TSP, 98 atom % D, Sigma-Aldrich, Milan) at a 0.75-mM final concentration, and transferred into a 5-mm NMR tube. A volume of 650 μL of the final solution was then transferred into a 5-mm NMR tube.
1H NMR experiments and data processing
1H NMR experiments were carried out on a Varian UNITY INOVA 500 spectrometer (Agilent Technologies, CA, USA) operating at 499.839 MHz. Spectra were acquired at 300K using the standard 1D NOESY pulse sequence for water suppression with a mixing time of 1 ms and a recycle time of 21.5 s. Spectra were recorded with a spectral width of 6000 Hz, a 90° pulse, and 128 scans. Spectra were processed using MestReNova software (Version 9.0, Mestrelab Research S.L.). Prior to Fourier transformation, the free induction decays (FID) were multiplied by an exponential weighting function equivalent to a line broadening of 0.5 Hz and zero-filled to 128K. All spectra were phased, baseline-corrected, and referenced to TSP at 0.00 ppm. The spectral region 0.80–9.00 ppm was segmented into buckets of 0.02 ppm width. The integrated area within each bin was normalised to a constant sum of 100 for each spectrum. The region containing the residual water resonance was excluded before integration. The assignment of the metabolites in the 1H NMR spectra was performed based on literature data [24, 27], the HMDB database (http://www.hmdb.ca) [28], the Chenomx NMR Suite 8.2 Library (Chenomx Inc., Edmonton, Canada), and comparison with spectra of standard compounds recorded using the same experimental conditions. Moreover, using the Chenomx NMR Suite Profiler tool, a set of 52 quantified metabolites was obtained (Supplementary Table S1). The final data set was exported as a text file for multivariate statistical data analysis. Mean centring was applied prior to performing data analysis.
CIA experiments for potassium determination
Standards and chemicals
Standard solutions of potassium (K+) and barium (Ba2+) were prepared from AnalaR salts (KCl and BaCl2) (Merck, Darmstadt, Germany). 18-Crown-6 ether (99% pure) and α-hydroxybutyric acid (HIBA) (99% pure) were obtained from Aldrich (Milan, Italy); imidazole (99% pure) and glacial acetic acid were obtained from Sigma (St. Louis, MO, USA). All chemicals were of analytical-reagent grade. Ultrapure water was obtained by an ELGA VEOLIA (Lane End, High Wycombe, UK) water purification system.
Instrumentation
All experiments were performed using a P/ACE MDQ Capillary Electrophoresis System (Beckman, Fullerton, CA, USA) equipped with a UV filter detector set at 214 nm wavelength, with indirect detection. In all the experiments, untreated fused-silica capillaries (75 μm I.D., 50 cm effective length; Beckman) were used. The capillary was thermostated at 25 °C. Beckman P/ACE Station (version 8.0) software was used for instrument control, data acquisition, and processing. Separations were performed as previously described [29]. Briefly, the running buffer was composed of 5 mM imidazole, 6 mM HIBA, and 5 mM 18-crown-6 ether adjusted to pH 4.5 with acetic acid. Constant voltage runs were performed in all experiments by applying a field of 500 V/cm. The analytes were injected at the anodic end of the capillary at 0.5 psi for 10 s. Prior to analysis, all samples were diluted 1:20 with a 40 μg/mL solution of BaCl2 (internal standard). Between consecutive runs, the capillary was washed with water (3 min) and then with the running buffer (2 min).
Statistical data analysis
Exploratory data analysis was performed by principal component analysis (PCA) to discover outliers and specific trends in the data. Thus, supervised data analysis based on Projection to Latent Structure (PLS) regression [30] was applied to evaluate the effects of PMI on the metabolomic profiles of the collected samples.
Since PMI showed a non-linear behaviour with respect to the metabolite content of VH, two different regression approaches were applied.
The first approach considered PMI as an ordinal variable after dividing the PMI window into intervals. Because the metabolite concentrations measured in VH were strongly correlated and the number of training samples was smaller than the number of predictors, standard regression methods developed for ordinal data such as ordered logistic regression could not be applied. For this reason, we developed a new method based on PLS. The method is a three-step procedure where in the first step the levels of the ordinal variable are used as classes to drive a PLS for a classification (PLS2-C) [31] model whose score components are used as predictors in the second step where post-transformation of PLS (pfPLS2) [30] is used to model the ordinal variable represented by means of the rank of its levels. As a result, an order between the levels of the ordinal variable is introduced. After these two steps, the latent variable explaining the ordinal variable is calculated. To transform the continuous latent variable into a categorical variable, a Naïve Bayes classifier is built. Since PLS techniques are robust in the presence of predictor matrices that are not full-column rank matrices, the method is suitable for metabolomics investigations. Repeated N-fold cross-validation was applied in model optimization to estimate the number of PLS components to use for PLS2-C and ptPLS2, and randomisation test to assess the reliability of the obtained model. A suitable cost function based on the misclassification error was minimised during cross-validation. Selectivity ratio (SR) [32] based on the predictive component of the ptPLS2 model was calculated to discover the metabolites mainly associated to PMI.
The second approach was developed to directly estimate PMI by regression. Since PMI has only positive values, we considered the logit-transformation to map PMI into a new space where regression was performed. Specifically, PMI was linearly mapped into the closed interval [ε1, ε2], being ε1 and ε2 two model parameters in ]0,1[ with ε2 > ε1 to be estimated from the data, and the images ε of PMI in [ε1, ε2] were logit-transformed to obtain the response to be submitted to PLS2 regression. PMI was, then, predicted applying the PLS2 model on the metabolic profile and using the logistic function to obtain the image ε of PMI in ]0,1[. That image was linearly transformed into PMI in the range [0,L] where L is the maximum value assumed for the prediction of PMI. The procedure assured that the predicted values were positive. Moreover, non-linearity could be introduced modifying the model parameters ε1 and ε2. Indeed, assuming ε1 and ε2 are close to 0.5, the model was linear whereas non-linearity was introduced for ε1 and ε2 moving towards 0 or 1. Parameters ε1 and ε2 and the number of PLS2 components were optimised by repeated N-fold cross-validation maximising the R2 calculated by cross-validation, i.e. Q2. A randomisation test was applied to test the reliability of the model. Model interpretation in terms of single predictors was performed calculating the SR parameter.
PCA was performed by SIMCA 17 (Sartorius Stedim Data Analytics AB) whereas suitable in-house functions implemented by R 4.0.4 platform (R Foundation for Statistical Computing) were built to perform PLS-based data analysis.
Results
VH metabolomic profile vs. PMI
VH samples (n = 71) collected at different PMIs, ranging from 6 to 84 h, were analysed by 1H NMR. The 47 samples selected for the training set and the remaining 24 samples used as test set covered the entire investigated PMI window.
Outlier detection was performed considering the centred binned data and applying the T2 Hotelling test and the Q-residual distance test. Assuming α = 0.05, no outliers were detected.
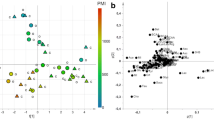
Exploratory data analysis by PCA applied to the autoscaled set of 52 quantified metabolites generated a model with 2 principal components, R2 = 0.488 and Q2 = 0.363. The score scatter plot and the loading plot are reported in Fig. 1. Colouring the samples in the score scatter plot (Fig. 1A) according to PMI shows a PMI-related trajectory of samples from the upper right-hand corner to the bottom left-hand corner, proving that the data variation of the quantified metabolites included information about PMI. Specifically, the investigation of the loading plot (Fig. 1B) allowed the identification of single metabolites mainly associated to PMI, as indicated in the caption of Fig. 1. After exploratory data analysis, regression modelling was applied. Firstly, the PMI temporal window was split into three intervals corresponding to PMI less than 24 h (interval A), PMI from 24 to 48 h (interval B), and PMI greater than 48 h (interval C), respectively, and an ordinal regression model based on PLS was built to predict the interval of PMI given the quantified metabolites. Samples resulted to be balanced with respect to the PMI intervals: interval A included 15 training samples and 7 test samples, and interval B included 14 training and 8 test samples, whereas 18 training and 9 test samples were included in interval C. Autoscaling the data, the model showed 3 components for the PLS2-C part and 2 components for the ptPLS2 part. The model passed the randomisation test (1000 random permutation) assuming α = 0.05. The results in calculating, cross-validating (20 repetitions of 5-fold cross-validation), and predicting the test set have been reported as confusion matrices in Fig. 2. It is worth noting that misclassification errors occurred only in contiguous intervals. In particular, when the test set is used, only two samples belonging to interval B were misclassified, one being predicted to belong to interval A and the other to interval C. Assuming α = 0.05, we found that alanine, creatine, succinate, glycine, hypoxanthine, choline, ethanolamine, glutamate, taurine, and 3-hydroxybutyrate resulted to be significantly related to PMI on the basis of SR. Only 3-hydroxybutyrate decreased with PMI whilst the other relevant metabolites were positively correlated to PMI. Thus, PMI was directly modelled using the new PLS approach developed for positive response and non-linear regression. The dataset of the quantified metabolites was log-transformed and autoscaled. The model showed 3 components, ε1=0.02, ε2=0.10, R2=0.941 (p < 0.001), and Q2=0.852 (p < 0.001). The model passed the randomisation test (1000 random permutation) assuming α = 0.05. The root mean square error (RMSE) in calculation was 5.6 h, RMSE by 20-repeated 5-fold cross-validation was 8.9 h, and RMSE obtained predicting the test set was 8.5 h. Considering the intervals defined in the case of ordinal regression, the RMSEs estimated using the test set were 6.9 h for PMI < 24 h, 7.4 h for PMI between 24 and 48 h, and 10.3 h for PMI > 48 h. Assuming α = 0.05, the analysis of the SR spectrum allowed us to discover threonine, alanine, glutamate, and glycine, which are positively correlated to PMI, and glucose and 3-hydroxybutyrate, which are negatively correlated to PMI, as significantly relevant. The results of the interpretation of the regression models are summarised in Fig. 3.
PCA model: PMI increases from the upper right-hand corner to the bottom left-hand corner (panel A). Investigating the loading plot (panel B), the decreasing of glucose, pyruvate, and 3-OH-butyrate with the increasing of PMI is observed, whilst late PMI samples (48–84 h) are characterised by higher levels of taurine, choline, creatine, hypoxanthine, ethanolamine, and succinate. Samples of the training set are indicated as “training” whereas samples of the test set as “test”
The SR obtained for the ordinal regression model (SRordinal) and that of the regression model (SR) are reported in the same plot; both the ordinal and the regression models discovered 3-hydroxybutyrate, negatively correlated to PMI, and alanine, glutamate, and glycine, positively correlated to PMI, as significantly relevant in predicting PMI (the profiles of these metabolites are reported in Fig. S2 of Supplementary Materials). The SR values have been multiplied by the sign of the Pearson correlation coefficient calculated between PMI and metabolite concentration; dashed red lines indicate the thresholds of SR at level α = 0.05
[K+] vs. PMI
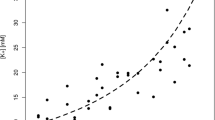
VH potassium concentrations [K+] were determined in 71 VH samples collected at different PMIs, ranging from 6 to 84 h. The potassium concentrations in the analysed samples ranged from 7.60 to 39.20 mM.
The relationship between [K+] and PMI was investigated by linear regression. The model showed R2=0.582 (p < 0.001), Q2=0.543 (p < 0.001), RMSE in calculation equal to 14.7 h, RMSE estimated by 20-repeated 5-fold cross-validation equal to 15.4 h, and RMSE in prediction equal to 11.5 h. Specifically, the RMSEs estimated using the test set were 14.2 h for PMI < 24 h, 8.8 h for PMI between 24 and 48 h, and 11.2 h for PMI > 48 h. In Fig. 4, we have reported in the same plot [K+] vs. PMI. The prediction errors resulted to be worse than those obtained considering the quantified metabolites. This is particularly relevant for PMI less than 24 h. However, potassium concentration may help in predicting PMI if considered together with metabolite concentration. As a proof-of-principle, we investigated the relationship between potassium concentration and quantified metabolites by PLS2 regression. Metabolic data were log-transformed and autoscaled. The PLS2 model showed 1 component, R2=0.631 (p < 0.001), and Q2=0.535 (p < 0.001). The metabolites that better explain [K+] were discovered by SR (Fig. 5). Considering the unexplained variance of [K+] and modelling PMI by linear regression, we obtained a model with R2=0.022 (p = 0.315) and Q2=− 0.097 (p = 0.760) that resulted to be unreliable. We could conclude that most of the data variation of the potassium concentration can be explained by the quantified metabolites and that the unexplained variance is not related to PMI. Therefore, the use of potassium concentration together with metabolite concentration may not lead to a relevant improvement in the modelling of PMI with respect to the use of the quantified metabolites alone.
Potassium concentration vs. quantified metabolites: SR plot; threonine, choline, alanine, hypoxanthine, taurine, creatine, glutamate, and glycine, positively correlated to [K+], and glucose and 3-hydroxybutyrate, negatively correlated to [K+], were discovered as relevant. The SR values have been multiplied by the sign of the Pearson correlation coefficient calculated between [K+] and metabolite concentration; dashed red lines indicate the thresholds of SR at level α = 0.05
VH metabolites and [K+] vs. PMI
The 52 quantified metabolites and the potassium concentration were considered to model PMI using the non-linear PLS regression approach. Metabolic data were log-transformed, and the potassium concentration was included as [K+]γ being the power γ estimated from the data. The parameters γ, ε1, and ε2 and the number of components of the PLS2 model were optimised maximising Q2 calculated by 20-repeated 5-fold cross-validation. The best model showed 2 components, γ = 3, ε1=0.015, ε2=0.10, R2=0.942 (p < 0.001), and Q2=0.871 (p < 0.001). The model passed the randomisation test (1000 random permutations) assuming α = 0.05. The RMSE in calculation was 5.5 h, the RMSE in cross-validation 8.4 h, and the RMSE in prediction 7.4 h. Specifically, the errors estimated using the test set were 5.7 h for PMI < 24 h, 7.4 h for PMI between 24 and 48 h, and 8.4 h for PMI > 48 h. In Table 1, we have summarised the results of the obtained models. As expected, the improvement obtained including potassium concentration in the set of the predictors is weak and mainly concerns PMI after 48 h. Moreover, [K+] is not significantly relevant if one considers SR and α = 0.05.
A tentative refinement of our model through the selection of the optimal subset which minimises the error in cross-validation (10 predictors instead of 53) leads to an error in prediction of 8.1 h compared to 8.4 h when all the 53 predictors are considered, namely a decrease of less than 5%. Moreover, considering each single relevant predictor, the errors in prediction increase more than 70% compared to the use of all predictors.
Discussion
VH has been long established as a biofluid of choice in forensic post-mortem investigation, with particular attention to PMI estimation. The work here presented is part of a wider project aimed at testing new methods for PMI estimation over different temporal windows in animal models. The rationale of our approach was based on the specific choice of biological matrices appropriate for the study of different PMI ranges. In particular, AH was successfully tested to accurately establish PMI up to 24 h after death, with a new approach combining 1H NMR metabolomic and potassium analysis [26]. Since AH cannot be usually sampled at higher PMIs, the approach was translated to VH which has been traditionally used for this purpose [13, 15].
The observed post-mortem VH metabolome modifications were exploited to build a predictive model for PMI and was compared with the model based on VH potassium obtained in the same dataset, being the latter the actual scientific gold standard for PMI estimation. In the hypothesis of investigating two different biological phenomena, the combined use of the two experimental approaches was also tested.
Results show that VH metabolomic composition is gradually modified both qualitatively and quantitatively with increasing PMIs. The best regression model for PMI estimation obtained using VH quantified metabolites is a non-linear model characterised by a high predictive ability; indeed, when tested on an independent set of VH samples, it shows an error in prediction of 8.5 h over the entire PMI range (80 h ranging from the 6th to the 86th h after death). When three selected PMI ranges are considered (< 24, 24–48, > 48 h), the errors in prediction increase with increasing PMI. This is foreseeable as both endogenous and exogenous inferences may cause a progressively increasing biological complexity, yielding to less detectable phenomena.
Another approach using sample classification over three selected PMI ranges appeared very robust, allowing 22 out of 24 samples to be correctly assigned to the proper range. Interestingly, the two misclassified samples belong to the intermediate range, and they occur between contiguous classes. This is consistent with the fact that modifications related to post-mortem interval are continuous, progressive, and developing phenomena.
Focusing on qualitative features of the VH metabolome, glycine, glutamate, alanine, creatine, choline, succinate, hypoxanthine, taurine, threonine, and ethanolamine showed a strong positive correlation with PMI, whereas 3-hydroxybutyrate and glucose were negatively correlated. Comparison of results coming from the two models clearly shows that several metabolites are responsible for most of the time-related differences. Considering that 3-hydroxybutyrate, alanine, glycine, and glutamate are shared between an ordinal and regression model, a major biological relevance can be inferred. The observed metabolomic modifications showed a linear behaviour over the entire PMI range, as no specific features can be identified in the three selected ranges.
The best PMI regression model obtained with VH potassium concentrations displays a lower predictive ability compared to metabolomics. The errors in predictions resulted higher over the entire PMI range mainly due to the error in the first 24 h, which tends to improve in later PMIs, reaching a predictivity with comparable metabolomics. This is consistent with literature data, suggesting human VH potassium as a valuable PMI estimation tool above 24 h after death (PMI ranging from 2 to 110 h) [29].
In the hypothesis that the two approaches inherently carry different or complementary biological information, we used a combined model. Whilst in early and middle PMI ranges the predictive ability is comparable to the one obtained by the sole metabolome, potassium contribution becomes noticeable in the longest PMI range (> 48 h). This can be explained by the fact that all the potassium variance is already included in the metabolome modifications up to 48 h. At higher PMIs, metabolome is likely influenced by exogenous bacterial contribution whereas potassium might still reflect the endogenous phenomenon.
As we previously reported in the AH model, a multivariate metabolomic approach better describes the multifactorial post-mortem phenomenon compared to the best univariate model (i.e. based on a single metabolite taken from the profile) [23, 26]. In particular, predictor selection did not show any significant improvement of our model based on 53 predictors—[K+] and 52 metabolites. Moreover, limiting the analysis to the best-performing predictors reduces the accounted inter-individual variability and could represent a bias for the prediction of new samples. This is extremely important in view of the translation of this model to real-life human forensic samples.
This study has several limitations. The investigation was conducted on an animal model, a highly homogeneous and controlled experimental setup being mandatory to precisely estimate PMIs. The translation to human cases represents the following step and should include real-life scenario, different environmental conditions, and multiple causes of death. Furthermore, VH was sampled from isolated sheep heads leading to a possible exogenous bacterial interference on both metabolome and potassium, although the model seems to be reliable and robust. Lastly, VH samples were delivered from the University of Cagliari, where the animal experiment was performed, to the University of Verona for potassium analysis; this may have influenced sample stability, and therefore the potassium CIA results, although the behaviour on the investigated range is consistent with literature data.
To the best of our knowledge, this study and the previous one on AH [26] proposed a tool for PMI estimation providing for the first time a PMI prediction model externally validated using an independent set of samples. This model validation strategy, which is a specific issue of potassium according to the literature [33], is mandatory to assess interlaboratory reproducibility, a fundamental step towards the implementation in routine casework.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Laplace K, Baccino E, Peyron PA (2021) Estimation of the time since death based on body cooling: a comparative study of four temperature-based methods. Int J Legal Med 135:2479–2487. https://doi.org/10.1007/s00414-021-02635-7
Wilk LS, Hoveling RJM, Edelman GJ, Hardy HJJ, van Schouwen S, van Venrooij H, Aalders MCG (2020) Reconstructing the time since death using noninvasive thermometry and numerical analysis. Sci Adv 6:eaba4243. https://doi.org/10.1126/sciadv.aba4243
Wilk LS, Edelman GJ, Roos M, Clerkx M, Dijkman I, Melgar JV, Oostra RJ, Aalders MCG (2021) Individualised and non-contact post-mortem interval determination of human bodies using visible and thermal 3D imaging. Nat Commun 12:5997. https://doi.org/10.1038/s41467-021-26318-4
Peyron PA, Hirtz C, Baccino E, Ginestet N, Tiers L, Martinez AY, Lehmann S, Delaby C (2021) Tau protein in cerebrospinal fluid: a novel biomarker of the time of death? Int J Legal Med 135:2081–2089. https://doi.org/10.1007/s00414-021-02558-3
Choi KM, Zissler A, Kim E, Ehrenfellner B, Cho E, Lee SI, Steinbacher P, Yun KN, Shin JH, Kim JY, Stoiber W, Chung H, Monticelli FC, Kim JY, Pittner S (2019) Postmortem proteomics to discover biomarkers for forensic PMI estimation. Int J Legal Med 133:899–908. https://doi.org/10.1007/s00414-019-02011-6
Peng D, Lv M, Li Z, Tian H, Qu S, Jin B, Long B, Liang W, Zhang L (2020) Postmortem interval determination using mRNA markers and DNA normalization. Int J Legal Med 134:149–157. https://doi.org/10.1007/s00414-019-02199-7
Sangwan A, Singh SP, Singh P, Gupta OP, Manas A, Gupta S (2021) Role of molecular techniques in PMI estimation: an update. J Forensic Leg Med 83:102251. https://doi.org/10.1016/j.jflm.2021.102251
De-Giorgio F, Ciasca G, Fecondo G, Mazzini A, De Spirito M, Pascali VL (2021) Estimation of the time of death by measuring the variation of lateral cerebral ventricle volume and cerebrospinal fluid radiodensity using postmortem computed tomography. Int J Legal Med 135:2615–2623. https://doi.org/10.1007/s00414-021-02698-6
De-Giorgio F, Ciasca G, D’Amico R, Trombatore P, D’Angelo A, Rinaldi P, Milano F, Locci E, De Spirito M, d’Aloja E, Colosimo C, Pascali VL (2020) An evaluation of the objectivity and reproducibility of shear wave elastography in estimating the post-mortem interval: a tissue biomechanical perspective. Int J Legal Med 134:1939–1948. https://doi.org/10.1007/s00414-020-02370-5
Palacio C, Gottardo R, Cirielli V, Musile G, Agard Y, Bortolotti F, Tagliaro F (2021) Simultaneous analysis of potassium and ammonium ions in the vitreous humour by capillary electrophoresis and their integrated use to infer the post mortem interval (PMI). Med Sci Law 61:96–104. https://doi.org/10.1177/0025802420934239
Ang JL, Collis S, Dhillon B, Cackett P (2021) The eye in forensic medicine: a narrative review. Asia Pac J Ophthalmol (Phila) 10:486–494. https://doi.org/10.1097/APO.0000000000000426
Tambuzzi S, Gentile G, Bailo P, Andreola S, Zoja R (2022) Use of cadaveric vitreous humor as an innovative substrate for diatoms research and forensic diagnosis of drowning. Int J Legal Med 136:1745–1754. https://doi.org/10.1007/s00414-021-02759-w
Cordeiro C, Ordóñez-Mayán L, Lendoiro E, Febrero-Bande M, Nuno Vieira D, Muñoz-Barús JI (2019) A reliable method for estimating the postmortem interval from the biochemistry of the vitreous humor, temperature and body weight. Forensic Sci Int. 295:157–168. https://doi.org/10.1016/j.forsciint.2018.12.007
Boroumand M, Grassi VM, Castagnola F, De-Giorgio F, d’Aloja E, Vetrugno G, Pascali VL, Vincenzoni F, Iavarone F, Faa G, Castagnola M (2022) Estimation of postmortem interval using top-down HPLC-MS analysis of peptide fragments in vitreous humour: a pilot study. Int J Mass Spectrom 483:116952. https://doi.org/10.1016/j.ijms.2022.116952
Pigaiani N, Bertaso A, De Palo EF, Bortolotti F, Tagliaro F (2020) Vitreous humor endogenous compounds analysis for post-mortem forensic investigation. Forensic Sci Int 310:110235. https://doi.org/10.1016/j.forsciint.2020.110235
Ferreira PG, Muñoz-Aguirre M, Reverter F, Sá Godinho CP, Sousa A, Amadoz A, Sodaei R, Hidalgo MR, Pervouchine D, Carbonell-Caballero J, Nurtdinov R, Breschi A, Amador R, Oliveira P, Çubuk C, Curado J, Aguet F, Oliveira C, Dopazo J et al (2018) The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat Commun 9:490. https://doi.org/10.1038/s41467-017-02772-x
Lyu L, Sonik N, Bhattacharya S (2021) An overview of lipidomics utilizing cadaver derived biological samples. Expert Rev Proteomics. 18:453–461. https://doi.org/10.1080/14789450.2021.1941894
Díez López C, Vidaki A, Kayser M (2022) Integrating the human microbiome in the forensic toolkit: current bottlenecks and future solutions. Forensic Sci Int Genet 56:102627. https://doi.org/10.1016/j.fsigen.2021.102627
Procopio N, Lovisolo F, Sguazzi G, Ghignone S, Voyron S, Migliario M, Renò F, Sellitto F, D’Angiolella G, Tozzo P, Caenazzo L, Gino S (2021) “Touch microbiome” as a potential tool for forensic investigation: a pilot study. Journal of Forensic and Legal Medicine 82:102223. https://doi.org/10.1016/j.jflm.2021.102223
Procopio N, Williams A, Chamberlain AT, Buckley M (2018) Forensic proteomics for the evaluation of the post-mortem decay in bones. Journal of Proteomics 177:21–30. https://doi.org/10.1016/j.jprot.2018.01.016
Locci E, Bazzano G, Chighine A, Locco F, Ferraro E, Demontis R, d’Aloja E (2020) Forensic NMR metabolomics: one more arrow in the quiver. Metabolomics 16:118. https://doi.org/10.1007/s11306-020-01743-6
Dawidowska J, Krzyżanowska M, Markuszewski MJ, Kaliszan M (2021) The application of metabolomics in forensic science with focus on forensic toxicology and time-of-death estimation. Metabolites 11:801. https://doi.org/10.3390/metabo11120801
Chighine A, Locci E, Nioi M, d’Aloja E (2021) Looking for post-mortem metabolomic standardization: waiting for Godot-the importance of post-mortem interval in forensic metabolomics. Chem Res Toxicol 34:1946–1947. https://doi.org/10.1021/acs.chemrestox.1c00211
Rosa MF, Scano P, Noto A, Nioi M, Sanna R, Paribello F, De-Giorgio F, Locci E, d’Aloja E (2015) Monitoring the modifications of the vitreous humor metabolite profile after death: an animal model. Biomed Res Int 2015:627201. https://doi.org/10.1155/2015/627201
Locci E, Stocchero M, Noto A, Chighine A, Natali L, Napoli PE, Caria R, De-Giorgio F, Nioi M, d’Aloja E (2019) A 1H NMR metabolomic approach for the estimation of the time since death using aqueous humour: an animal model. Metabolomics 15:76. https://doi.org/10.1007/s11306-019-1533-2
Locci E, Stocchero M, Gottardo R, De-Giorgio F, Demontis R, Nioi M, Chighine A, Tagliaro F, d’Aloja E (2021) Comparative use of aqueous humour 1H NMR metabolomics and potassium concentration for PMI estimation in an animal model. Int J Legal Med 135:845–852. https://doi.org/10.1007/s00414-020-02468-w
Locci E, Scano P, Rosa MF, Nioi M, Noto A, Atzori L, Demontis R, De-Giorgio F, d’Aloja E (2014) A metabolomic approach to animal vitreous humor topographical composition: a pilot study. PLoS One 9:e97773. https://doi.org/10.1371/journal.pone.0097773
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J et al (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Research 46:608–617. https://doi.org/10.1093/nar/gkx1089
Bortolotti F, Pascali JP, Davis GG, Smith FP, Brissie RM, Tagliaro F (2011) Study of vitreous potassium correlation with time since death in the postmortem range from 2 to 110 hours using capillary ion analysis. Med Sci Law. 51(Suppl 1):S20–S23. https://doi.org/10.1258/msl.2010.010063
Stocchero M (2019) Iterative deflation algorithm, eigenvalue equations, and PLS2. J. Chemometr. 33:e3144. https://doi.org/10.1002/cem.3144
Stocchero M, De Nardi M, Scarpa B (2021) PLS for classification. Chemometrics Intell Lab Syst 216:104374. https://doi.org/10.1016/j.chemolab.2021.104374
Kvalheim OM (2010) Interpretation of partial least squares regression models by means of target projection and selectivity ratio plots. J. Chemometr. 24:496–504
Ortmann J, Markwerth P, Madea B (2016) Precision of estimating the time since death by vitreous potassium-comparison of 5 different equations. Forensic Sci Int. 269:1–7. https://doi.org/10.1016/j.forsciint.2016.10.005
Funding
Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors. As sheep heads represent waste material, there were neither need of an ad hoc animal protocol nor associated cost.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials 1:
Supplementary Table S1. List of ovine VH metabolites quantified using the Chenomx Profiler tool. Fig. S1 Values of PMI predicted for the test set (PMI predicted) vs measured values (PMI): predictions from the model obtained considering the quantified metabolites (panel A), the vitreous potassium concentration (panel B) and the combination of quantified metabolites and potassium concentration (panel C); the diagonal is reported in the plots. Fig. S2 Profiles of the significantly relevant metabolites discovered by both ordinal and regression models: 3-hydroxybutyrate (panel A), alanine (panel B), glutamate (panel C) and glycine (panel D); orthogonal scaling has been applied to concentration to have the same scale [-1,1] for all the metabolites; dashed lines indicate the linear regression lines
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Locci, E., Stocchero, M., Gottardo, R. et al. PMI estimation through metabolomics and potassium analysis on animal vitreous humour. Int J Legal Med 137, 887–895 (2023). https://doi.org/10.1007/s00414-023-02975-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-02975-6