Abstract
Purpose
The objective of this study is to systematically review the international literature for dynamic sleep magnetic resonance imaging (MRI) as a diagnostic tool in obstructive sleep apnea (OSA), to perform meta-analysis on the quantitative data from the review, and to discuss its implications in future research and potential clinical applications.
Study design
A comprehensive review of the literature was performed, followed by a detailed analysis of the relevant data that has been published on the topic.
Methods
Clinical key, Uptodate, Ovid, Ebscohost, Pubmed/MEDLINE, Scopus, Dynamed, Web of Science and The Cochrane Library were systematically searched. Once the search was completed, dynamic sleep MRI data were analyzed.
Results
Nineteen articles reported on 410 OSA patients and 79 controls that underwent dynamic sleep MRI and were included in this review. For meta-analysis of dynamic sleep MRI data, eight articles presented relevant data on 160 OSA patients. Obstruction was reported as follows: retropalatal (RP) 98%, retroglossal (RG) 41% and hypopharyngeal (HP) in 5%. Lateral pharyngeal wall (LPW) collapse was found in 35/73 (48%) patients. The combinations of RP + RG were observed in 24% and RP + RG + LPW in 16%. If sedation was used, 98% of study participants fell asleep compared to 66% of unsedated participants.
Conclusions
Dynamic sleep MRI has demonstrated that nearly all patients have retropalatal obstruction, retroglossal obstruction is common and hypopharyngeal obstruction is rare. Nearly all patients (98%) who are sedated are able to fall asleep during the MRI. There is significant heterogeneity in the literature and standardization is needed.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a well-recognized and commonly occurring condition in which an individual suffers frequent apneic episodes causing arousal from sleep leading to fragmented and poor quality of sleep [1]. There is an increased risk in all-cause mortality of those affected by moderate to severe OSA [2]. The polysomnography (PSG) has been the gold standard for the diagnosis of OSA and the severity is graded by the apnea hypoxia index (AHI) calculated by the number of apneas or hypopneas per hour of sleep [2]. Patients then usually undergo treatment with continuous positive airway pressure (CPAP) or other medical management such as oral appliances, myofunctional therapies, and/or nasopharyngeal airway devices if indicated [3, 4]. However, if patients cannot tolerate medical management, or if their OSA is severe enough that medical management is ineffective, patients will often seek surgical treatment. There are many options for surgical treatments including soft tissue surgeries, as well as skeletal surgeries and hypoglossal nerve stimulator implants that have proven effective for the management of OSA [5]. However, with many surgical options available, it is important to delineate which surgeries would be most effective for each patient. Assessment tools include physical examination, drug-induced sleep endoscopy (DISE), awake fiberoptic laryngoscopy with Muller’s maneuver, and imaging including videofluoroscopy, cephalometry, computed tomography (CT), and magnetic resonance imaging (MRI). Each method has its advantages and disadvantages.
Imaging of the soft tissues in the upper airway for the purposes of OSA is typically acquired either in the form of CT or MRI has evolved through time. In 1993, Suto et al. was the first to publish on using a novel form of MRI that was capable of creating video MRI images with ultrafast “FLASH” (Fast low angle shot) technology [6]. Multiple slices in the midsagittal and axial planes are acquired over approximately 30 s during a breath cycle or apneic event. The resulting series can show the duration, degree as well as the pattern of airway collapse [7, 8]. This type of MRI study has been reported by many different names in the literature including video MRI, cine-MRI, FLASH MRI, ultrafast MRI, and dynamic MRI, but for the purposes of this study, it will be referred to as a “dynamic MRI” study. MRI done in this fashion can be conducted during natural sleep, although many studies are done under induced sleep. More recent studies use dynamic MRI in the awake state to study upper airway changes during tidal breathing.
MRI used in the traditional technique has also been used in the study of OSA patients and will be reported as “static MRI”. Static MRI is a useful tool for identifying anatomical differences between patients with and without OSA [9–12]. Volumetric data calculated from static MRI of the soft palate, tongue, lateral wall volumes, and parapharyngeal fat pads can be predictors of OSA severity [9, 13–16]. On the other hand, dynamic sleep MRI has potential to be applied clinically by providing images of the exact sites and pattern of obstruction in OSA patients while asleep.
This article provides a literature review and meta-analysis for dynamic sleep MRI techniques as a diagnostic tool for OSA. It reviews and analyzes dynamic sleep MRI findings regarding the pattern and sites of obstruction. The study methods and protocol for falling sleep in the MRI setting are reviewed and standardization for future studies are outlined. Finally, the article discusses the advantages and disadvantages of using dynamic sleep MRI as a diagnosis tool, future areas of research, and its potential in clinical applications.
Methods
The preferred reporting items for systematic reviews and meta-analysis (PRIMSA) statement was followed in the preparation and writing of this article [17].
Search strategy
Clinical key, Uptodate, Ovid, Ebscohost, Pubmed/MEDLINE, Scopus, Dynamed, Web of Science and The Cochrane Library were systematically searched by authors KV, SC, and CM independently from inception through April 1, 2020. One example of a search strategy used in PubMed/MEDLINE is: (“MRI” OR “Magnetic resonance imaging”) AND (“sleep” OR “obstructive” OR “Apnea” OR “Apnea”) AND (“dynamic” OR “video” OR “cine” OR “FLASH” OR “ultrafast”). Each search was reviewed, and full text-pdf versions of articles were cataloged if determined to be potentially relevant.
Study selection
Studies that were included for review were all those that included patients that had OSA and a dynamic MRI was performed in a sleep state either by natural or induced sleep. Studies on pediatric patients were excluded. Studies without dynamic MRI were excluded, and studies that performed awake only MRI were excluded.
Studies that were included for meta-analysis were those from the review that had data on site of obstruction seen on dynamic sleep MRI. Studies were excluded if the site or pattern of obstruction could not clearly be elucidated by the authors. If it could not be determined that the cohort was not previously published, the most recent or most relevant study was included for meta-analysis and the others excluded.
Data abstractions and study quality assessment
The authors searched all available databases for relevant articles. A consensus was made as to which studies to include in this analysis and the final decision was made by author MC. The Data collected from the articles included the number of patients, gender, age, body mass index (BMI), apnea–hypopnea index (AHI), sedation use, region of collapse seen on the imaging study, and whether the participant fell asleep in the MRI machine. If the average and standard deviation was provided, it was calculated from the reported data. The studies identified in this analysis were all case series and were assessed using the National Institute for Health and Clinical Excellence (NICE) quality assessment tool [18].
Statistical analysis
StatsToDo.com was used for totaling averages and standard deviations. If data were missing, the totaling was not performed, or the study was not included in the calculation. If studies were missing data, the plan was to contact the corresponding author at least twice so the data could potentially be obtained.
Results
Systematic review
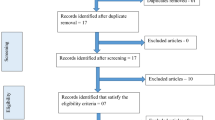
The literature search yielded 635 articles that were screened and 50 articles that were downloaded in full text pdf for review. After applying exclusion criteria, 19 articles were included for systematic review. Figure 1 shows the literature review flow diagram. Please see online supplementary Table 1 for details of the NICE quality assessment, but in summary seven articles scored 7, six articles scored 6, two articles scored 5, two articles scored 4, one article scored 3, and one scored 1. Nine articles had controls groups, and four had BMI-matched controls. Details regarding each article in the review including a synopsis and brief discussion of each can be found on Table 1.
Meta-analysis
After systematic review, 8 articles provided data on dynamic sleep MRI that were included for meta-analysis (Table 2). Two articles (Liu et al. and Huon et al.) were noted to have reported the same data set, therefore the more recent article was included for analysis [19, 20]. Bansal et al. was not included in the analysis due to a paucity of details regarding the patients AHI, BMI, and observations of sites of obstruction [21]. Wang et al. used dynamic imaging for 41 patients under natural sleep but the data were not included in the meta-analysis as the authors reported on intrinsic changes of the tongue during sleep rather than the sites of obstruction [22]. Shintani et al. reported elements of anterior–posterior measurements and cross-sectional areas in different levels of the upper airway rather than obstructions and was not easily adapted to this meta-analysis [23]. Huon et al. reported on both mild and severe OSA patients, thus that data were split for the purposes of meta-analysis [20]. In total 160 OSA patients (AHI 38.7 events/hr + / − 26.6, BMI 27.2 kg/m2 + / − 4.6) and 25 controls were included. Moon et al. reported RDI and was included in the AHI calculation [24].
The average BMI of the OSA patients was 27.2 kg/m2 + / − 4.6 which is in the overweight category according to the Centers for Disease Control and Prevention. 131 patients were male and 19 were female; the gender was not reported for 10 patients in study by Chuang et al. [25]. The average age was 47.8 years old, standard deviation was not reported in 3 studies and unable to be calculated en mass.
The collapse and obstruction were visualized in 5 regions: nasopharyngeal (NP), retropalatal (RP), retroglossal (RG), hypopharyngeal (HP), and lateral pharyngeal wall (LPW). Different terminologies were used across the literature in reporting the sites of obstruction (i.e., velopharyngeal, soft palate, retropalatal, retrolingual, retroglossal, hypopharynx, epiglottic, epipharynx, mesopharynx, tongue base). The descriptions of these regions were able to be consolidated in this meta-analysis. The NP region is defined as the air space above the hard palate. RP is defined superiorly from an extension from the hard palate to the inferior border of the uvula [20, 26, 27]. RG is defined from inferior border of the uvula to the superior border of the epiglottis [6, 24, 26–28]. Some studies extended RG to the base of epiglottis, however a HP region were not defined in those studies [20, 25]. Future studies in correlating with DISE would be able to easily identify the tip of the epiglottis rather than the base, thus the definition using the tip was favored. Of note, retrolingual and retroglossal are semantically identical, however, a majority of studies used retroglossal and thus this term was adapted in this analysis. The HP region is defined as the air space below the superior tip of the epiglottis. HP collapse was presumably due to epiglottic collapse, although this is not specified [13, 40]. LPW collapse was not reported in a majority of the studies as this would require axial slices in multiple planes combined with coronal slices for optimal visualization. Huon et al. were the only authors to report specifically on LPW collapse, in part because they included coronal cuts in the study. Chuang et al. and Moon et al. were able to characterize “circumferential” or transverse collapse on axial imaging, which was interpreted as LPW collapse [24, 25].
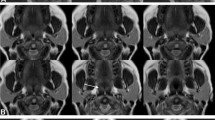
Obstruction, also synonymous with collapse or narrowing, was defined differently in among studies. Obstruction in the majority of the studies was defined as the disappearance of the airspace in the RP or RG area in the sagittal, and axial planes if applicable [26, 28, 29]. Moon et al. and Barerra et al. defined a > 50% reduction in length in the sagittal plane and whereas Huon et al. defined a > 75% reduction in length in the sagittal plane as obstruction [20, 24, 30]. Lee et al. used a classification of no obstruction, partial obstruction, and complete obstruction [31]. A 50% reduction in length, if assumed to be circumferential, would increase airflow resistance by a factor of 16 according to Poiseuille law [32]. Kavcic et al. reported 25% RG narrowing using specific measurements in the axial plane as obstruction if associated with an apneic event, whereas Berrera et al. reported a 50% change in RG anterior–posterior dimension as a positive obstruction as judged by the two interpreters without using specific measurements to quantify a 50% change [26, 30]. The subjective interpretation of obstructions was shown to have good inter-rater reliability in the RP area, but some variability was reported in the RG area [30]. Obstruction in the meta-analysis was adapted using the criteria as defined by each study. Figure 2 demonstrates an example of RP and RG obstruction as seen on imaging. NP obstruction was not seen in any patient. RP obstruction was found in 157 of 160 (98.1%) patients, RG obstruction was found in 65/160 (40.6%) patients, and HP obstruction was found in 8/160 (5%) patients. LPW collapse was only recorded in studies including axial or coronal planes and was found in 35/73 (48%) patients. Multi-level obstructions were also reported. The combination of RP + RG was found in 38/160 (23.8%) patients. The combination of RP + RG + LPW was found in 25/73 (34.2%) patients. Nine patients exhibited RP and LPW, 4 patients exhibited RP + HP, and 3 patients were found to have RP + RG + HP (Table 2).
This image demonstrates (A) Patent airway (B) Retropalatal obstruction with retroglossal narrowing (C) Retropalatal obstruction without retroglossal narrowing. All images taken on midline sagittal view using dynamic sleep MRI. This image is from Sleep magnetic resonance imaging with electroencephalogram in obstructive sleep apnea syndrome, Kavcic et al. [26], reprinted with permission from Wiley
In a separate analysis, all articles in the review were assessed for ability of the patients to fall asleep in the MRI machine. In 19 articles, 526 participants attempted sleep and 406 (77.19%) were able to fall asleep in total. Eleven articles reported using natural sleep for the MRI. Nearly all describe some form of sleep deprivation period prior to attempting the scan. These periods ranged from 12 to 20 h, and in those reports the patients were not allowed alcohol or sedatives prior to the procedure. In total, natural sleep was attempted in 364 patients and successful sleep, defined differently in studies, was achieved in 240 patients reflecting a 65.93% success rate of falling asleep in an MRI machine. Seven articles reported using a myriad of sedatives ranging from zolpidem, hydroxyzine, midazolam, chloral hydrate, or propofol to achieve sleep state. In the induction group, 169 participants attempted sleep, and 166 (98.17%) were able to achieve sleep (Fig. 3). The only patients reported that did not achieve sleep with induction agents were in one report by Zhang et al. that used chloral hydrate as the agent [8]. This cohort was also the only one to attempt natural sleep, and then if unable was given the sedative; these patients were counted twice (once as attempting natural sleep, and once as attempting sedated sleep if given the sedative). Midazolam and hydroxyzine were each used in two articles, propofol and zolpidem were each used in one. Suto et al. did not specify induction vs natural in their cohort, but likely reported the same cohort as a previous publication by the same author group that used hydroxyzine as the induction agent; this data were categorized as induced sleep [6, 33]. Bansal et al. was unique in that it was the only study conducted after lunch, in which the patients ingested a heavy meal to induce sleep, however an unspecified “low dose oral sedative” was also given [21]. See online supplementary Table 2 for details.
Discussion
OSA is a pervasive problem with many different treatment options both surgical and non-surgical. While PSG remains the gold standard for diagnosis of OSA, it is limited in providing information regarding the area of obstruction. CPAP remains the gold standard for initial treatment; however, for those patients with severe cases, or those patients who otherwise opt for surgical intervention, it is important to determine a targeted therapy. Considering that there are numerous surgical interventions targeting different areas in the upper airway, determining the specific area of obstruction will be key in surgical planning. Dynamic sleep MRI presents itself as an intuitive way to view these specific areas of obstruction, and it is the first documented diagnostic modality that can image the upper airway during natural sleep [6]. To have a more thorough understanding of dynamic sleep MRI, multiple topics require further discussion. These topics include the nuances and correlations between different areas of obstruction and its clinical application, as well as detailed review of the sub-group analysis regarding the feasibility of natural and induced sleep. Finally, the use of dynamic sleep MRI with other adjunct to study sleep, the limitations of the dynamic sleep MRI technique, and comparative imaging modalities are explored.
LPW, RP, and RG obstructions have some notable correlates. The pattern of RP and RG obstruction was not able to be correlated with severity of AHI. However, in the study by Huang et al., LPW collapse was positively correlated with significantly higher AHI [20] than those without LPW collapse. In our study, the RP area was the most prevent area of obstruction found in 98.7% of subjects. There were instances of isolated RP obstruction, but there were no instances of isolated RG obstruction; in other words, RG obstruction was always associated with another area of obstruction. RP obstruction occurring independently of as well as in combination with RG narrowing was observed in the same patient during different apneas [26]. Findings by Kavcic et al. noted narrowing of the RG space if the soft palate was attached to the tongue base and no narrowing of the RG space if the soft palate was detached to the tongue base [26]. The theorized mechanism proposed by the authors related to the increased surface tension of the soft palate to the base of tongue causing posterior displacement. Chuang et al. similarly described three different sequences of obstruction between the soft palate and tongue base: (1) Posterior movement of the soft palate without tongue movement, (2) RP obstruction followed by the RG obstruction, and (3) Tongue displacement posteriorly first to compress the soft palate causing obstruction [25]. Lee et al., using CT instead of MRI, has observations relevant to this discussion [31]. They described two types of RP collapse: uvular type (lower part of the soft palate and uvula collapse) and velar type (lower soft palate collapse occurring first, followed by upper soft palate collapse). They also defined 3 types of tongue collapse: upper type (tongue body moving backwards to compress the soft palate and narrow the UA), lower type (tongue base/lingual tonsils moving backwards to contact the posterior pharyngeal wall and narrow the UA), and upper plus lower type (upper tongue collapse occurring first, followed by lower tongue collapse) for the tongue [31]. Such detailed classification of RG and RP obstruction was not available for the meta-analysis but should be acknowledged that not all RG obstruction or RP obstruction are identical. In clinical correlate, if a hypoglossal nerve stimulator was used for a patient who had isolated RP collapse, the treatment could be less effective than a for a patient with combined RP and RG collapse, in that instance a procedure targeting the palate might be more applicable. Patient outcomes based on surgical intervention targeted on areas of obstruction pre-operative dynamic imaging is an area of ongoing research. For example, dynamic sleep MRI to assess obstruction in patient with multilevel obstruction or non-responders prior to complex airway surgery has been reported in a small cohort of patients. Surgical outcomes of the small sample size 6 months postoperatively showed varied success in AHI reduction highlighting the complex interaction that occurs in the soft tissues of the upper airway [34].
A major advantage of the sleep MRI is the ability to image the airway during natural sleep; however, some patients may not be able to fall asleep in the machine. Techniques such as FLASH and ultrafast MRI have shortened the duration of the scan, sequences may be adjusted to decrease the sound pressure, white noise headphones or earplugs have been used with success [20, 26], and performing the studies in the evening at a normal bedtime with patients staying awake throughout the day may help provide conditions for natural sleep. Ikeda et al. advocated for awake instead of asleep dynamic MRI stating that most patients cannot fall asleep in the machine during natural sleep [29]. However, our study finds that the majority, 240 of 364 (65.93%) participants that attempted natural sleep were able to fall asleep. It must be stated that each study setting was unique and small sample size from the studies limits a conclusion regarding successful natural sleep in an MRI. In addition, these numbers are greatly subject to reporting bias, as one study by Liu et al. showed in a consecutive series of 159 patients, only 64 (40.25%) fell asleep naturally [19]. Other studies that were not reported consecutively or that may not have reported the number of patients excluded for not falling asleep, may falsely elevate the natural sleep success rate. In contrast, when patients were induced with some form of pharmacologic induction agent 166 of 169 patients (98%) were able to fall asleep in the scanner. A higher success rate with induced sleep may increase the efficacy of the dynamic MRI scans, however, sedation may alter the muscular tone of the upper airway and exaggerate the degree of airway collapse when compared to natural sleep [19, 26, 35]. This has a potential for over diagnosis of obstructive sites, especially in the RG region. In addition, respiratory depression after sedation is a significant risk in OSA patients due to the inhibitory effects on ventilatory drive and the confined space inside an MRI may restrict access to the airway in the event of over sedation [31, 35]. There were no comments of complications or airway compromise in the studies utilizing medication-induced sleep. More research would need to be conducted on the selection and dose of sedative that would be optimal for dynamic sleep MRI in order achieve a high rate of successful sleep without disrupting normal oropharyngeal muscle tone and physiology.
The study of OSA with dynamic MRI can be assisted and enhanced by additional studies. Baseline anatomic findings such as BMI, grading of tonsil and adenoid hypertrophy, and Friedman anatomic stage are important in establishing patient characteristics. The VOTE classification with DISE, which describes the configuration of collapse as A-P, lateral, or concentric, can also be correlated with axial images being taken at the narrowest point in the RP and RG areas (which correlating to the inferior tip of the uvula and the superior tip of the epiglottis) [36]. Preliminary dynamic sleep MRI results have shown to be highly correlated to DISE findings in the RP area although correlation in the RG area was not statistically significant [27]. In addition, PSG while in MRI, Peripheral arterial tone (PAT), and Electroencephalogram (EEG) studies can assist with monitoring the quality and stage of sleep during which the dynamic MRI images are obtained. Upper airway muscle tone and obstruction sites may be different in progressive stages of sleep. Studies including EEG noted that patients are only able to reach light sleep (stages 1 or 2) on EEG during the short duration of the scan [26]. Other factors that may contribute to the pathophysiology of OSA such as intraluminal pressure, pharyngeal air flow, upper airway resistance, and muscle laxity are not able to be detected with imaging alone [37]. In the pediatric population, dynamic MRI has been used in conjunction with computational fluid dynamics, and pneumotachography to measure air flow rates to further characterize the upper airway motion [38]. Wu et al. used face mask and pressure measurement devices simultaneously with MRI scan to measure airway compliance; the study concluded that the sites of airway narrowing did not always correspond to higher collapsibility [39]. Epworth sleepiness scale (ESS), Nasal Obstruction and Septoplasty Effectiveness (NOSE) scale and other quality of life measures would be important to prior to any interventions prompted by sleep MRI findings to correlate with symptomatic improvement in the patient. The MRI images can also be investigated using novel computerized analysis techniques. For example, using a “tagged” technique, the amount of soft tissue deformation can be quantified. This method has been described in identifying different compartments of the tongue and revealed higher OSA severity associated with movement of a greater number of tongue compartments [40]. Areas of concurrent research using dynamic MRI includes the use in the awake state as a potential screening tool for severe OSA. A BMI-matched cohort study revealed significantly narrower RP airway in OSA patients [41]. In addition, awake axial images at the 4th cervical vertebral level have been used to calculate minimum airway ellipticity as a predictor for severe OSA [42]. However, the site of narrowing does not always correspond to higher collapsibility when asleep [39]. Finally, comparison of pre- and post-surgical dynamic sleep MRI can help the surgeon better understand the dynamic interactions altered by any surgical interventions. The potential for the surgeon to visualize post-surgical changes and locate new or persistent sites of obstruction could be invaluable. It may reveal the possible etiology for only marginal improvement in AHI in some patients for certain procedures and guide further advancements in surgical interventions.
Dynamic sleep MRI has its limitations in terms of acquisition technique as well as cost. Patient positioning while acquiring the images may pose as a challenge as the patient must be comfortable, yet motionless. In studies by Barrera, patients were allowed to lay however they felt most comfortable and the use of lateral head supports was not specified [30, 43, 44]. Small rotational movements of the head may go unnoticed causing misalignment of the midsagittal plane which could lead to incorrect diagnosis of obstruction on imaging [45]. Finally, the cost of an MRI varies widely by location and institution. A 2 h sleep MRI has been reported to cost approximately 1000$ [30]. A component to the cost is due to the duration needed for natural sleep to occur; however, it may be offset with scanning overnight during non-peak usage times.
In comparison to other imaging techniques, CT imaging has been used in a similar manner to visualize obstructive patterns at multiple levels in the upper airway during sleep [31, 46]. It is less expensive, quiet, and timely. However, the distinction between MRI and CT is radiation exposure and type of images produced with MRI offering better soft tissue resolution. While providers are working to reduce radiation from CT using different techniques or reducing the duration of the scan, the risk for radiation-induced cancer with even low dose radiation must be considered. The MRI findings are corroborated by CT studies done in a similar manner comparing awake vs sleep states revealing upper airway narrowing during apneic events predominantly at the RP level rather than RG region [47].
The observations and conclusions of this retrospective review are limited to the quality of the studies that have been published previously. Overall, there is a great deal of heterogeneity in the current literature. Lack of robust, consecutive studies that included age and BMI-matched controls with large sample size adhering to a standardized protocol create limitations in the data for meta-analysis. Only 2 studies had BMI-matched non-OSA controls [29, 48]. The effects of obesity in correlation with OSA are well studied and the use of BMI-matched non-OSA patients in future studies are imperative in further delineating the collapse seen in OSA patients. Multiple articles in the systematic review were unable to be included in the meta-analysis due to the heterogeneity of the reported data. A sub-group analysis could not be performed for sites of obstruction, BMI, AHI, age, etc. due to unavailable individual patient data in most studies. The majority of patients included in the meta-analysis were males with severe OSA and a majority of the studies were of Asian ethnicity. Ethnic difference in upper airway anatomy between Europeans and Chinese OSA patients has been reported [49]. The effects of these differences in reference to dynamic collapse are unknown.
Conclusion
Dynamic sleep MRI is an exciting adjunct for the evaluation of OSA patients and determining the specific locations and patterns of obstruction. This study corroborates the finding that RP collapse is the most prevalent finding in males with severe OSA. Dynamic sleep MRI can be done under natural sleep, although there is a higher efficacy with various sedation methods. For control groups in future studies, it is imperative to include BMI-matched non-OSA patients. In addition, measurement of airway obstructions in a standardized approach with standardized nomenclature of the levels of obstruction (NP, RP, RG, HP, and LPW) will allow correlation between observances. Along with sagittal images, axial and coronal images would need to be utilized to determine more precise patterns of collapse and especially elucidate the extent of LPW collapse.
Potentially, if able to overcome these challenges, dynamic sleep MRI could be applied to a broader clinical practice to guide appropriate surgical decision making for severe OSA patient with multilevel collapse or refractory to initial surgical intervention.
Data availability
All data are available within the manuscript or by contact with the corresponding author.
Code availability
Not applicable.
References
Wright J, Johns R, Watt I, Melville A, Sheldon T, Royal B et al (1997) Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ 314:851–869
Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, George T et al (2009) Sleep-disordered breathing and mortality : a prospective cohort study. PLoS Med 6(8):e1000132
Camacho M, Certal V, Abdullatif J, Zaghi S, Ruoff CM, Capasso R (2014) Myofunctional therapy to treat obstructive sleep apnea : a systematic review and meta-analysis. Sleep 38:669–675
Kumar AR, Guilleminault C, Certal V, Li D, Capasso R, Camacho M (2015) Nasopharyngeal airway stenting devices for obstructive sleep apnoea : a systematic review and. J Laryngol Otol 129:2–10
Camacho M, Certal V, Capasso R (2013) Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol [Internet] Associação Brasileira de Otorrinolaringol e Cirurgia Cérvico-Facial 79:780–788. https://doi.org/10.5935/1808-8694.20130139
Suto Y, Matsuo T, Kato T, Hori I, Inoue Y, Ogawa S et al (1993) Evaluation of the pharyngeal airway in patients with sleep apnea : value of ultrafast MR imaging. Am J Roentgenol 160:311–314
Fleck RJ, Shott SR, Mahmoud M, Ishman SL, Amin RS, Donnelly LF (2018) Magnetic resonance imaging of obstructive sleep apnea in children. Pediatr Radiol 48(9):1223–1233
Zhang X, Yang X, Hua H, Chen J (2009) Comparison of MRI fast SPGR single slice scan and continuous dynamic scan in patients with obstructive sleep apnea – hypopnea syndrome. Eur J Radiol 71:17–21
Chi L, Mitra N, Reilly MP, Wan F, Maislin G, Chmiewski L et al (2011) Identification of craniofacial risk factors for obstructive sleep apnoea using three- dimensional MRI. Eur Respir J 38:348–358
Li Y, Lin N, Ye J, Chang Q (2012) Upper airway fat tissue distribution in subjects with obstructive sleep apnea and its effect on retropalatal mechanical loads. Respir Care 57:1098–1105
Stuck BA, Neff W, Hörmann K, Verse T, Bran G, Baisch A et al (2005) Anatomic changes after hyoid suspension for obstructive sleep apnea : an MRI study. Otolaryngol Neck Surg 133:397–402
Fricke BL, Donnelly LF, Shott SR, Kalra M, Poe SA, Chini BA (2006) Comparison of lingual tonsil size as depicted on MR imaging between children with obstructive sleep apnea despite previous tonsillectomy and adenoidectomy and normal controls. Pediatr Radiol 36:518–523
Ciscar MA, Juan G, Martinez V, Ramon M, Lloret T, Minguez J et al (2001) Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J 17:79–86
Pahkala R, Seppä J, Ikonen A, Smirnov G, Tuomilehto H (2014) The impact of pharyngeal fat tissue on the pathogenesis of obstructive sleep apnea. Sleep Breath [Internet] 18:275–282. https://doi.org/10.1007/s11325-013-0878-4
Turnbull CD, Wang SH, Manuel AR, Keenan BT, Mcintyre AG, Schwab RJ et al (2018) Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath 22(3):673–681
Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R et al (2003) Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric MRI. Am J Respir Crit Care Med 168:522–530
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses : the PRISMA statement. PLoS Med [Internet] 6:1–8. https://doi.org/10.1136/bmj.b2535
(2012) Methods for the de development of NICE public health guidance [Internet]. Third NatlInst Heal Clin Excell. www.nice.org.uk. Accessed 17 May 2020
Liu S, Huon L, Lo M, Chang Y, Capasso R, Chen Y et al (2016) Static craniofacial measurements and dynamic airway collapse patterns associated with severe obstructive sleep apnoea: a sleep MRI study. Clin Otolaryngol 41:700–706
Huon L, Liu SY, Shih TT, Chen Y, Lo M, Wang P et al (2016) Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Oto-Rhino-Laryngol 273:4021–4026
Bansal R, Sahu A, Rastogi D, Sachdeva S, Aggarwal B (2018) Dynamic MRI evaluation of the neck for levels of airway obstruction in the patients with obstructive sleep apnoea.
Wang Y, Mcdonald JP, Liu Y, Pan K, Zhang X, Hu R (2014) Dynamic alterations of the tongue in obstructive sleep apnea-hypopnea syndrome during sleep : analysis using ultrafast MRI. Genet Mol Res 13:4552–4563
Shintani T, Kozawa T, Himi T (2003) Obstructive sleep apnea by analysis of MRI findings. Int Congr Ser 1257:99–102
Moon IJ, Han DH, Kim J, Rhee C, Sung M-W, Park J-W et al (2010) Sleep magnetic resonance imaging as a new diagnostic method in obstructive sleep apnea syndrome. Laryngoscope 120:2546–2554
Chuang L-P, Chen N-H, Li H-Y, Lin S-W, Chou Y-T, Wang C-J et al (2009) Dynamic upper airway changes during sleep in patients with obstructive sleep apnea syndrome. Acta Otolaryngol 129:1474–1479
Kavcic P, Koren A, Koritnik B, Fajdiga I, Groselj LD (2015) Sleep magnetic resonance imaging with electroencephalogram in obstructive sleep apnea syndrome. Laryngoscope 125:1485–1490
Gamaleldin O, Bahgat A, Anwar O, Seif-Elnasr M, Eissa L, Razek AAKA et al (2020) Role of dynamic sleep MRI in obstructive sleep apnea syndrome. Oral Radiol [Internet]. https://doi.org/10.1007/s11282-020-00455-w
Moriwaki H, Inoue Y, Namba K, Suto Y, Chiba S, Moriyama H (2009) Clinical significance of upper airway obstruction pattern during apneic episodes on ultrafast dynamic magnetic resonance imaging. Auris Nasus Larynx 36:187–191
Ikeda K, Ogura M, Higano S, Hida W, Oshima T, Takahashi S et al (2001) Quantitative assessment of the pharyngeal airway by dynamic magnetic resonance imaging in obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol 110:183–189
Barrera JE (2011) Sleep magnetic resonance imaging : dynamic characteristics of the airway during sleep in obstructive sleep apnea syndrome. Laryngoscope 121:1327–1335
Lee L-A, Wnag C-J, Lo Y-L, Huang C, Kuo I-C, Lin W-N et al (2019) Drug-induced sleep computed tomography-directed upper airway surgery for obstructive sleep apnea: a pilot study. Otolaryngol Neck Surg 160:172–181
Pfitzner J (1976) Poiseuille and his law. Anaesthesia England 31:273–275
Suto Y, Ohmura N, Inoue Y, Ohta Y (1997) Technical note: reconstruction of three-dimensional images of the pharynx in patients with sleep apnea using three-dimensional fast low-angle shot MR imaging. Am J Roentgenol 168:1320–1321
Faria AC, Garcia LV, Santos AC, Eckeli AL, Garcia DM, Mello-filho FV (2016) Dynamic comparison of pharyngeal stability during sleep in patients with obstructive sleep apnea syndrome treated with maxillomandibular advancement. Sleep Breath 21:25–30
Den HC, Schmeck J, Appelboom DJK, De VN (2004) Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ 329:955–959
Kezirian EJ, Hohenhorst W, de Vries N (2011) Drug-induced sleep endoscopy: the VOTE classification. Eur Arch oto-rhino-laryngology Off J Eur Fed Oto-Rhino-Laryngological Soc Affil with Ger Soc Oto-Rhino-Laryngology-Head Neck Surg Germany 268:1233–1236
Osman AM, Carter SG, Carberry JC, Eckert DJ (2018) Obstructive sleep apnea: current perspectives. Nat Sci Sleep 10:21–34
Bates AJ, Schuh A, Mcconnell K, Williams BM, Lanier JM, Loew W, et al. (2017) Combining computational fluid dynamics and 3d-cine mri to determine the relationship between upper airway motion and breathing effort in pediatric obstructive sleep apnea.
Wu Z, Chen W, Khoo MCK, Ward SLD, Nayak KS (2016) Evaluation of upper airway collapsibility using real-time MRI. J Magn Reson Imaging 44:158–167
Juge L, Knapman FL, Burke PGR, Brown E, De FAFB, Gandevia SC et al (2020) Regional respiratory movement of the tongue is coordinated during wakefulness and is larger in severe obstructive sleep apnoea The Journal of Physiology. J Physiol 3:581–597
Feng Y, Keenan BT, Wang S, Leinwand S, Wiemken A, Pack AI et al (2018) Dynamic upper airway imaging during wakefulness in obese subjects with and without sleep apnea. Am J Respir Crit Care Med 198:1435–1443
Kojima T, Kawakubo M, Nishizaka M, Rahmawati A, Ando S, Akiko C et al (2018) Assessment by airway ellipticity on cine-MRI to differentiate severe obstructive sleep apnea. Clin Respir J 12:878–884
Barrera JE, Holbrook AB, Santos J, Popelka GR (2009) Sleep MRI: novel technique to identify airway obstruction in obstructive sleep apnea. Otolaryngol Neck Surg 140(3):423–425. https://doi.org/10.1016/j.otohns.2008.11.037
Barrera JE, Chang RC, Popelka GR, Holbrook AB (2010) Reliability of airway obstruction analyses from Sleep MRI sequences. Otolaryngol Neck Surg 142(4):526–530. https://doi.org/10.1016/j.otohns.2010.01.003
Shin LK, Holbrook AB, Capasso R, Kushida CA, Powell NB, Fischbein NJ et al (2013) Improved sleep MRI at 3 tesla in patients with obstructive sleep apnea. J Magn Reson Imaging 38:1261–1266
Li H, Lo Y, Wang C, Hsin L, Lin W, Fang T et al (2016) Dynamic drug-induced sleep computed tomography in adults with obstructive sleep apnea. Sci Rep [Internet] 6:1–8. https://doi.org/10.1038/srep35849 (Nature Publishing Group)
Passos UL, Genta PR, Marcondes BF (2019) State-dependent changes in the upper airway assessed by multidetector CT in healthy individuals and during obstructive events in patients with sleep apnea. J Bras Pneumol 45:1–9
Darquenne C, Elliott AR, Sibille B, Smales ET, Deyoung PN, Theilmann RJ et al (2018) Upper airway dynamic imaging during tidal breathing in awake and asleep subjects with obstructive sleep apnea and healthy controls. Physiol Rep 6:1–9
Xu L, Keenan BT, Wiemken AS, Chi L, Staley B, Wang Z et al (2019) Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep 43(5):273
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government. The authors have no funding, financial relationships, or conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript was submitted and accepted for oral presentation at the Combined Otolaryngology Spring Meetings, Triological Society, Atlanta, GA, USA, April 22–26 (canceled due to SARS-CoV2).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Volner, K., Chao, S. & Camacho, M. Dynamic sleep MRI in obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 279, 595–607 (2022). https://doi.org/10.1007/s00405-021-06942-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06942-y