Abstract
Purpose
The use of autologous tissues is considered gold standard for patients undergoing breast reconstruction and is the preferred method in the post-radiation setting. Although the latissimus dorsi flap (LDF) has been replaced by abdominal flaps as technique of choice, it remains a valuable option in several specific clinical situations and its use has been regaining popularity in recent years. In this work, we present an 18-year retrospective analysis of a single-institution single-surgeon experience with LDF-based reconstruction with focus on early complications and reconstructive failures.
Methods
Hospital records of all patients undergoing breast surgery for any reason in the Certified Breast Cancer Center, Regio Klinikum Pinneberg, Germany between April, 1st 2005 and October, 31st 2022 were reviewed. 142 consecutive LDF-based reconstructive procedures were identified. Detailed information was gathered on patient characteristics, treatment-related factors, and complications.
Results
One hundred forty patients (139 female, 1 male) received 142 LDF-based surgeries. The flap was used mainly for immediate breast reconstruction with or without implant (83% of patients), followed by defect coverage after removal of a large tumor (7%), implant-to-flap conversion with or without placement of a new implant (6%), and delayed post-mastectomy reconstruction (4%). The use of LDF decreased between 2005 and 2020 (2005: 17, 2006: 13, 2007: 14, 2008: 16, 2009: 5, 2010: 9, 2011: 8, 2012: 3, 2013: 10, 2014: 8, 2015: 8, 2016: 7, 2017: 7, 2018: 4, 2019: 4, 2020: 2, 2021: 6, 2022: 4). Surgery was performed for invasive breast cancer in 78%, ductal carcinoma in situ in 20% and other reasons such as genetic mutation in 1% of patients. Ipsilateral radiation therapy was received by 12% of patients prior to LDF surgery and by 37% after the surgery. 25% of patients were smokers. The median duration of surgery, including all procedures conducted simultaneously such as e.g., mastectomy, axillary surgery, or implant placement, was 117 min (range 56–205). Patients stayed in the hospital for a median of 7 days (range 2–23 days). The most common complication was seroma (26%), followed by wound dehiscence (8%), surgical site infection (7%), partial skin and/or nipple necrosis of any size (7%) and hematoma requiring surgical evacuation (2%). 19% of all patients required seroma aspiration or drainage, mostly at the donor site and performed under ultrasound guidance in the ambulatory setting. Flap loss due to necrosis occurred in 2% of patients.
Conclusions
Latissimus dorsi flap is a well-established surgical technique commonly used for immediate breast reconstruction as well as defect coverage in locally advanced breast cancer. To the best of our knowledge, this is one of the largest single-surgeon analyses of early complications in patients receiving LDF. As expected, seroma was the most common complication observed in nearly one third of patients and requiring a therapeutic intervention in every fifth patient. Serious adverse events occurred rarely, and flap loss rate was very low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This analysis confirms latissimus dorsi flap-based breast surgery as a robust technique with a low complication rate. The most common complication is the development of a seroma and flap loss is a rare occurrence. |
Introduction
The use of autologous tissues is considered gold standard for patients undergoing breast reconstruction, and is the preferred method in the post-radiation setting [1, 2]. Several procedures are available including pedicled flaps such as latissimus dorsi flap (LDF) and transverse rectus abdominis myocutaneous flap (TRAM), as well as free flap requiring microvascular anastomoses like the deep inferior perforator flap (DIEP). Some of these, like LDF, may be combined with implants to provide adequate volume and symmetry. In patients with limited soft tissue following mastectomy, breast reconstruction may involve several steps, e.g., tissue expander placement before positioning a permanent implant, surgery of the contralateral breast, or nipple and areola reconstruction.
While LDF is one of the oldest described muscle flaps, it did not gain wide recognition until the 1970s [3]. In the following years, numerous variations were introduced, such as de-epithelialized flap for volume replacement and extended flap including lumbar fat to maximize tissue amount [4, 5]. Although LDF has been replaced by abdominal flaps as autologous tissue of choice for breast reconstruction in most countries, it remains a reliable alternative and a valuable option in several specific clinical situations [6]. Its advantages are the robust vascular supply making flap necrosis a rare event, and, in case of combination approach, lower rates of capsular contracture compared to implant-only based reconstruction [7,8,9,10].
Although the use of LDF is regaining popularity in recent years [7, 8], evidence on complication rates and outcomes is limited. The 2015 German S3 guideline on breast reconstruction with autologous tissue identified only 22 articles on LDF-based reconstruction that reported on complication rates, the largest including 78 flaps [11]. In this work, we present an 18-year retrospective analysis of a single-institution single-surgeon experience with LDF-based reconstruction with a focus on early complications and reconstructive failures.
Material and methods
In this retrospective study, clinical records of all patients undergoing breast surgery for any reason in the Certified Breast Cancer Center, Regio Klinikum Pinneberg, Germany between 01.04.2005 and 30.10.2022 were reviewed. 142 latissimus dorsi flap (LDF) reconstructive procedures were identified. The hospital electronic record was then reviewed for patient-, tumor- and treatment-related information. The following information were included in the database: age, body mass index (BMI), smoking habits, concomitant diseases and medication, timing of reconstruction, previous breast surgery, type of breast and axillary surgery, surgery duration, simultaneous use of an implant, complications, and secondary surgeries were included in the database. The protocol version 1.1 of this retrospective analysis has been reviewed by the Ethical Committee of the Medical Board Schleswig–Holstein (27/02/19).
Statistical analysis
Chi-squared test and Fisher’s exact test were used to evaluate the relationship between complication rates and surgical parameters. All reported p-values are two-sided. Statistical analysis was performed by SPSS, version 18 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics, diagnosis, surgical setting, and previous and subsequent oncological treatments for the 142 LDF procedures performed in 140 patients are presented in Table 1. All procedures were performed by the same surgeon (P.P.). All procedures were performed as open surgery and neither endoscopic nor robotic approach was used. The humeral insertion of the latissimus dorsi muscle was transected in all cases [12]. All patients received single-shot antibiotic prophylaxis. Extended antibiotic prophylaxis was not used. Mean age was 61 and the median follow-up was 38.4 months. 25% of patients were smokers at time of surgery. Invasive breast cancer was the main leading diagnosis accounting for 78% of the cases (Table 1). 139 patients were female and only 1 patient was male. 138 patients received a unilateral LDF surgery and in two cases, LDF was used for both sides, albeit not simultaneously: (1) 38-year-old patient undergoing a mastectomy with immediate LDF- and implant reconstruction for locally advanced breast cancer was diagnosed with a contralateral multicentric breast cancer 8 years later and received skin-sparing mastectomy with LDF and implant; (2) 83-year-old patient undergoing a radical mastectomy for an ulcerated 19 cm large tumor with LDF coverage was diagnosed with extended contralateral cutaneous metastases 4 months later and underwent a mastectomy with wide-excision of all skin manifestations combined with LDF defect coverage. The number of procedures per year decreased during the period analyzed from 41 surgeries performed between 2005 and 2007 to 12 between 2020 and 2022 (Fig. 1, Supp. Table 1).
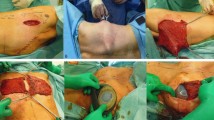
118 patients (83%) received immediate reconstruction, either with (n = 82) or without (n = 36) implant placement (Fig. 2, Table 1). The median volume of the inserted implant was 230 ml (range, 95–560 ml). In five patients, LDF surgery was performed for delayed reconstruction after mastectomy. Ten patients required LDF for defect coverage after removal of an advanced tumor (Fig. 3), and in nine cases LDF surgery was performed as revision procedure due to complications after previous surgery (e.g., implant-to-flap conversion in case of capsular fibrosis or wound healing disorders). Median duration of surgery, defined as time between first incision and final skin closure, was 117 min, including all additional procedures such as full axillary lymph node dissection (ALND, performed in 45 cases), sentinel lymph node biopsy (SLNB, 43 cases), implant removal and/or placement, removal of the port system, contralateral surgery, nipple reconstruction etc. The shortest duration of surgery was 56 min (83-year-old patient with breast cancer metastasizing to the bone receiving a palliative radical mastectomy for a large ulcerated breast cancer with defect coverage using an LDF flap). Comparing different clinical settings, the shortest median duration of surgery was observed in patients receiving LDF surgery for defect coverage (median 79 min). Patients remained in the hospital for a median of 7 days (range: 2–23 days). Most patients received at least one surgery of the ipsilateral breast prior to LDF procedure (range: 1–6). 12% underwent ipsilateral radiation therapy before and 37% after the surgery.
Complications following LDF surgery
Observed complications are summarized in Table 2. The most common complication was a seroma occurring in 26% of procedures. 19% of patients received at least one needle aspiration for seroma (range: 1–6) and in one case revision surgery with an easy flow drain placement was necessary. Total flap loss due to complications was observed in three cases (2.1%). Revision surgery, defined as a second surgery for any reason within 30 days of LDF procedure, was performed in 13 out of 142 (9.1%) patients. Details on these patients are shown in Table 3. Previous application of chemotherapy did not affect complication rate, but previous ipsilateral radiation therapy was associated with significantly higher rates of infection (24% vs. 5%, p = 0.005). Lower rates of infection and wound dehiscence were observed in patients receiving primary reconstruction, compared to delayed reconstruction and defect coverage. Higher number of previous breast surgeries was associated with higher rates of nipple and/or skin necrosis (p = 0.010) and hematoma (p = 0.020).
Follow up
The mean follow up was 38.4 months (range 0–200 months). None of the patients reported twitching or animation deformity of the flap. Out of 93 patients receiving an implant simultaneously with LDF surgery, 24 (26%) underwent implant removal. The median time of implant remaining in situ was 151.2 months (95% CI 90.8–211.6). The implants were mostly exchanged due to capsular fibrosis. In three cases the implant was permanently removed without replacement: in two cases the implant was removed due to invasive recurrence of the thoracic wall after 19 and 96 months, respectively, and in one case it was removed due to capsular contraction following radiation therapy without placement of a new implant (patient’s wish). The rate of implant removal and/or exchange in the long-term follow up was numerically higher in patients receiving pre- or postoperative radiation therapy but the difference was not statistically significant (32.4% vs. 21.8%, p = 0.256). The time point of radiation therapy was not significantly associated with implant removal and/or exchange (42.9% in case of radiation therapy before LDF surgery vs. 29% in case of postoperative radiation therapy).
Discussion
To the best of our knowledge, this is the largest single-surgeon report on clinical characteristics as well as complications in patients undergoing latissimus dorsi flap-based surgery of the breast. In contrast to other publications, which mostly reported on delayed LDF reconstructions, the majority of patients in our study received LDF in an immediate reconstruction setting directly following a mastectomy. [7, 13].
The most common early complication was a seroma, occurring in 26% of patients. This is in line with previous reports and some authors suggested that asymptomatic seroma should not be considered a complication but rather an inevitable side effect of LDF surgery [11, 14]. The current practice of electrocautery dissection may further contribute to development of symptomatic or recurrent seromas, and it is not uncommon to leave drains for several days or even weeks at the donor site. Tomita et al. analyzed potential risk factors for seroma development in 174 patients with LDF surgery [15]. Seroma occured in 28% of patients receiving mastectomy and LDF, while the incidence was much lower (11%) in patients undergoing a breast conserving surgery with LDF-based volume replacement. Higher age and BMI significantly increased the risk of seroma formation. In our study, age (p = 0.045) but not overweight were correlated to seroma development. Different approaches to prevent seroma formation have been explored in previous studies. Lee et al. performed a meta-analysis of 14 studies, including three randomized controlled trials, on quilting sutures and fibrin sealants [16]. Both interventions contributed in varying degrees to reducing seroma-related morbidity following LDF transfer, and their combination might have a synergistic effect. However, more than half of the included studies had a retrospective and non-randomized design and the definition of seroma varied. Further, operative time required for either intervention, additional costs, and the potential for allergic reaction to sealants need to be considered. Therefore, neither intervention is accepted as standard-of-care and the decision to use them depends on surgeon’s preference. In the present study, the prevalence of seroma was comparable to that reported in previous studies, even though no intervention for seroma prevention was used. While drain placement is commonly used in LDF surgery, recommendations regarding the timepoint of drain removal vary. Some authors recommend removing the last drain when drainage volume decreased under a specific amount (e.g., 20, 30 or 40 ml) per 24 h, while others prefer to leave the drains for a defined time, irrespective of the output [17, 18]. In the present analysis, drains were removed once the drainage volume decreased to < 30 ml in 24 h.
The most serious complication in the setting of flap-based reconstructive procedures is a partial or total flap loss, usually occurring due to disrupted perfusion and subsequent necrosis. While total flap loss has been observed in 1–2% of patients across studies, partial flap necrosis may occur in up to 9% of cases [11] (Table 4). In our study, we observed a total flap loss rate of 2%. No patient suffered from partial flap ross. Thus, these results confirm LDF surgery as a safe and robust method with a low failure risk.
Arm and shoulder morbidity and—in case of a simultaneous implant placement—capsular contracture, are considered typical late complications of LDF based surgery. In the setting of immediate or delayed reconstruction, LDF usually requires a combination with an implant to achieve desired breast size, due to the limited volume of the flap itself. In the present study, 26% of patients who received an implant required implant removal during follow-up. In most cases, this was followed by an immediate placement of a new implant (implant exchange). The reported implant removal or exchange rate in studies with longer follow-up may be as high as 50% [22,23,24]. Rates on the prevalence of capsular fibrosis following combined LDF and implant reconstruction vary widely among studies. Hardwicke et al. examined 277 consecutive LDF procedures combined with a placement of a textured cohesive gel implant, focusing on late complications [7]. After a mean follow-up of 47 months, higher-grade capsular contracture, defined as Baker III or IV, occurred in 3.6% of patients, resulting in a capsulotomy in all patients. Interestingly, administration of a chemotherapy was associated with a lower rate of capsular contracture, while no association with radiation therapy was seen. Similarly, in our analysis the administration of radiation therapy was not associated with implant removal rate in the long-term follow up. The mean time to first implant exchange was 22.4 months and thus shorter than in the present study.
Giacalone et al. reported on the long-term clinical follow-up of 104 patients in two cohorts: 26 patients received an immediate reconstruction using a LDF and prosthesis (either a tissue expander or a permanent implant) after neoadjuvant chemotherapy and 78 a delayed reconstruction after chemo- and radiation therapy [21]. Early implant loss occurred more frequently in the delayed reconstruction setting (12% vs. 0%), whereas late complications were more common after immediate reconstruction (30% vs. 21%). Capsular contracture rates were similar in both cohorts (15.3% after immediate and 11.5% after delayed reconstruction, respectively). The prevalence of capsular contracture increases with time [24].
In the present work no arm and shoulder-related problems were documented after a median follow-up of 38 months. However, data on shoulder impairment were not prospectively collected and no measurements of shoulder strength performed. Therefore, underreporting cannot be excluded. Altogether, data on arm and shoulder morbidity after LDF surgery vary across studies [11, 24, 25]. According to a meta-analysis of 26 studies, there is a tendency that the LDF transfer may affect shoulder function, but this limitation seems to be minimal, and few patients experience a major impact on shoulder function [26].
Regarding the surgical technique, two aspects need to be considered. First, it has been shown that transection of the tendinous insertion of the latissimus dorsi muscle on the humerus may improve aesthetic results [27]. Specifically, it helps to avoid the development of the displeasing bulge that patients often refer to as “carrying a book” under the armpit and may increase the mobility of the flap [27]. In the present study, the humeral insertion of the muscle was transected in all cases. Second, transecting the thoracodorsal nerve has been controversially discussed in the literature [12, 28,29,30,31]. In the present study, a simple transection of the thoracodorsal nerve was performed in all patients, which reflects the standard approach followed by most surgeons in Germany [27, 28, 32]. While leaving the nerve intact may lead to unintentional muscle twitching and breast animation, sometimes referred to as “jumping breast” phenomenon, some authors suggested that cutting the nerve may result in a decrease of flap volume and proposed an individual approach depending on the setting and the complexity of nerve identification [29, 30]. Interestingly, several studies reported that reinnervation may occur after simple nerve transection and that reinnervation rates may be reduced when larger nerve segments are resected [30].
The present study shows a slow decrease in the use of LDF-based breast reconstruction. A similar trend is currently being observed world-wide. Leff et al. examined immediate breast recontructions performed in the United Kingdom [33]. After an initial increase in the use of the technique observed between 1996 and 2008, the number of procedures decreased rapidly. At the same time, the use of other techniques of autologous tissue transfer increased from 0.44% in 1996 to 2.76% in 2012. A similar trend was noted in the United States as well [34,35,36]. Various reasons have been proposed to explain the decreased use of LDF procedures. First, the relatively low volume of the harvested flap makes a simultaneous use of a breast prosthesis necessary in most cases, which makes the technique unsuitable for patients opting for an implant-free reconstruction. Second, a worldwide trend towards bilateral mastectomy is observed [37, 38]. While LDF-based reconstruction of both breasts is possible, arm and shoulder morbidity after a bilateral procedure remains a concern. Finally, the improved access to abdominal flap transfer with microvascular anastomosis at high-volume centers makes pedicled flaps less attractive.
Conclusions
The present study confirms latissimus dorsi flap to be a safe and robust method for reconstruction of the breast and skin replacement of chest wall. As in previous studies, the leading early complication was a seroma, while serious complications such as total flap loss were a rare occurrence. Future studies should focus on the long-term evaluation of arm and shoulder morbidity as well as patient satisfaction with the aesthetic outcome.
References
Recommendations of the AGO Breast Committee (2022) Diagnosis and treatment of patients with early and advanced Breast Cancer, www.ago-online.de. Accessed 15 Aug 2023
(2021) NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, Version 2.2021—March 12, 2021, NCCN.org
Sood R, Easow JM, Konopka G, Panthaki ZJ (2018) Latissimus dorsi flap in breast reconstruction: recent innovations in the workhorse flap. Cancer Control. https://doi.org/10.1177/1073274817744638
Papp C, McCraw JB (1998) Autogenous latissimus breast reconstruction. Clin Plast Surg 25:261–266
Hokin JA, Silfverskiold KL (1987) Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg 79:58–66
Durkin AJ, Pierpont YN, Patel S, Tavana ML, Uberti MG, Payne WG, Smith DJ, Smith PD (2010) An algorithmic approach to breast reconstruction using latissimus dorsi myocutaneous flaps. Plast Reconstr Surg 125:1318–1327. https://doi.org/10.1097/PRS.0b013e3181d6e7b8
Hardwicke JT, Prinsloo DJ (2011) An analysis of 277 consecutive latissimus dorsi breast reconstructions: a focus on capsular contracture. Plast Reconstr Surg 128:63–70. https://doi.org/10.1097/PRS.0b013e3182174133
Sternberg EG, Perdikis G, McLaughlin SA, Terkonda SP, Waldorf JC (2006) Latissimus dorsi flap remains an excellent choice for breast reconstruction. Ann Plast Surg 56:31–35. https://doi.org/10.1097/01.sap.0000186463.07617.6f
Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A (2006) Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg 59:1017–1024. https://doi.org/10.1016/j.bjps.2006.03.057
Chang DW, Barnea Y, Robb GL (2008) Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg 122:356–362. https://doi.org/10.1097/PRS.0b013e31817d6303
(2015) National German Guideline (S3): Breast reconstruction with autologous tissue, AWMF Registry No. 015/075
Gerber B, Krause A, Reimer T, Muller H, Friese K (1999) Breast reconstruction with latissimus dorsi flap: improved aesthetic results after transection of its humeral insertion. Plast Reconstr Surg 103:1876–1881. https://doi.org/10.1097/00006534-199906000-00011
Palve J, Luukkaala T, Kaariainen M (2022) Comparison of different techniques in latissimus dorsi breast reconstruction: plain, immediately lipofilled, and implant enhanced. Ann Plast Surg 88:20–24. https://doi.org/10.1097/SAP.0000000000002798
Daltrey I, Thomson H, Hussien M, Krishna K, Rayter Z, Winters ZE (2006) Randomized clinical trial of the effect of quilting latissimus dorsi flap donor site on seroma formation. Br J Surg 93:825–830. https://doi.org/10.1002/bjs.5434
Tomita K, Yano K, Masuoka T, Matsuda K, Takada A, Hosokawa K (2007) Postoperative seroma formation in breast reconstruction with latissimus dorsi flaps: a retrospective study of 174 consecutive cases. Ann Plast Surg 59:149–151. https://doi.org/10.1097/SAP.0b013e31802c54ef
Lee KT, Mun GH (2015) Fibrin sealants and quilting suture for prevention of seroma formation following latissimus dorsi muscle harvest: a systematic review and meta-analysis. Aesthetic Plast Surg 39:399–409. https://doi.org/10.1007/s00266-015-0476-x
Menke H, Erkens M, Olbrisch RR (2001) Evolving concepts in breast reconstruction with latissimus dorsi flaps: results and follow-up of 121 consecutive patients. Ann Plast Surg 47:107–114. https://doi.org/10.1097/00000637-200108000-00001
Schwabegger A, Ninkovic M, Brenner E, Anderl H (1997) Seroma as a common donor site morbidity after harvesting the latissimus dorsi flap: observations on cause and prevention. Ann Plast Surg 38:594–597. https://doi.org/10.1097/00000637-199706000-00005
Paillocher N, Florczak AS, Richard M, Classe JM, Oger AS, Raro P, Wernert R, Lorimier G (2016) Evaluation of mastectomy with immediate autologous latissimus dorsi breast reconstruction following neoadjuvant chemotherapy and radiation therapy: a single institution study of 111 cases of invasive breast carcinoma. Eur J Surg Oncol 42:949–955. https://doi.org/10.1016/j.ejso.2016.03.024
Houvenaeghel G, El Hajj H, Schmitt A, Cohen M, Rua S, Barrou J, Lambaudie E, Bannier M (2020) Robotic-assisted skin sparing mastectomy and immediate reconstruction using latissimus dorsi flap a new effective and safe technique: a comparative study. Surg Oncol 35:406–411. https://doi.org/10.1016/j.suronc.2020.09.022
Giacalone PL, Rathat G, Daures JP, Benos P, Azria D, Rouleau C (2010) New concept for immediate breast reconstruction for invasive cancers: feasibility, oncological safety and esthetic outcome of post-neoadjuvant therapy immediate breast reconstruction versus delayed breast reconstruction: a prospective pilot study. Breast Cancer Res Treat 122:439–451. https://doi.org/10.1007/s10549-010-0951-7
Clarke-Pearson EM, Lin AM, Hertl C, Austen WG, Colwell AS (2016) Revisions in implant-based breast reconstruction: how does direct-to-implant measure up? Plast Reconstr Surg 137:1690–1699. https://doi.org/10.1097/PRS.0000000000002173
Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K (2014) The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg 134:588–595. https://doi.org/10.1097/PRS.0000000000000523
Tarantino I, Banic A, Fischer T (2006) Evaluation of late results in breast reconstruction by latissimus dorsi flap and prosthesis implantation. Plast Reconstr Surg 117:1387–1394. https://doi.org/10.1097/01.prs.0000207396.22527.68
Button J, Scott J, Taghizadeh R, Weiler-Mithoff E, Hart AM (2010) Shoulder function following autologous latissimus dorsi breast reconstruction. A prospective three year observational study comparing quilting and non-quilting donor site techniques. J Plast Reconstr Aesthet Surg 63:1505–1512. https://doi.org/10.1016/j.bjps.2009.08.017
Steffenssen MCW, Kristiansen AH, Damsgaard TE (2019) A Systematic review and meta-analysis of functional shoulder impairment after latissimus dorsi breast reconstruction. Ann Plast Surg 82:116–127. https://doi.org/10.1097/SAP.0000000000001691
Wallwiener D, Jonat W, Kreienberg R, Friese K, Diedrich K, Beckmann MW (2009) Atlas der gynäkologischen Operationen, 7th edn. Georg Thieme Verlag, Stuttgart
Abdallah A (2009) Onkoplastische Brustchirurgie. Deutscher Ärzte-Verlag, Köln
Kaariainen M, Giordano S, Kauhanen S, Helminen M, Kuokkanen H (2014) No need to cut the nerve in LD reconstruction to avoid jumping of the breast: a prospective randomized study. J Plast Reconstr Aesthet Surg 67:1106–1110. https://doi.org/10.1016/j.bjps.2014.04.029
Lopez CD, Kraenzlin F, Frost C, Darrach H, Aravind P, Sacks JM (2019) Latissimus denervation: a review of evidence. J Reconstr Microsurg 35:609–615. https://doi.org/10.1055/s-0039-1688748
Jones GE (2020) Latissimus dorsi flap reconstruction. Bostwick’s plastic and reconstructive breast surgery. Volume II: part VII non-microsurgical breast reconstruction. Georg Thieme Verlag, Stuttgart
Aktas B, Banys-Paluchowski M, Ditsch N. 2023 Atlas der onkoplastischen Brustoperationen (in press): Elsevier
Leff DR, Bottle A, Mayer E, Patten DK, Rao C, Aylin P, Hadjiminas DJ, Athanasiou T, Darzi A, Gui G (2015) Trends in immediate postmastectomy breast reconstruction in the United Kingdom. Plast Reconstr Surg Glob Open 3:e507. https://doi.org/10.1097/GOX.0000000000000484
Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, Cordeiro PG, Matros E (2013) A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 131:15–23. https://doi.org/10.1097/PRS.0b013e3182729cde
Hernandez-Boussard T, Zeidler K, Barzin A, Lee G, Curtin C (2013) Breast reconstruction national trends and healthcare implications. Breast J 19:463–469. https://doi.org/10.1111/tbj.12148
Jagsi R, Jiang J, Momoh AO, Alderman A, Giordano SH, Buchholz TA, Kronowitz SJ, Smith BD (2014) Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol 32:919–926. https://doi.org/10.1200/JCO.2013.52.2284
Findlay-Shirras L, Lima I, Smith G, Clemons M, Arnaout A (2021) Canada follows the US in the rise of bilateral mastectomies for unilateral breast cancer: a 23-year population cohort study. Breast Cancer Res Treat 185:517–525. https://doi.org/10.1007/s10549-020-05965-z
Shaheen MS, Momeni A (2022) Nationwide trends in contralateral prophylactic mastectomies: an analysis of 55,060 unilateral breast cancer patients. Plast Reconstr Surg Glob Open 10:e4344. https://doi.org/10.1097/GOX.0000000000004344
Acknowledgements
The authors would like to thank Mrs. Clarissa Lück for her excellent assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MB-P: project development, data management, data analysis, manuscript writing; LB: Project development, data collection and management, manuscript writing; NK: manuscript editing; SVK: data management, manuscript editing; MLG: data management, manuscript editing; NB: manuscript editing; AR: data management, manuscript editing; LH: data management, manuscript editing; FH: data management, manuscript editing; PP: project development, data management, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
M. Banys-Paluchowski received honoraria for lectures and advisory boards from: Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Pintuition, Pierre Fabre, ExactSciences, and study support from: EndoMag, Mammotome, MeritMedical, Gilead, Hologic, ExactSciences. Other authors declare no conflicts of interest.
Ethical approval
The protocol of this retrospective analysis has been reviewed and approved by the Ethical Committee of the Medical Board Schleswig–Holstein (27/02/19).
Consent to participate
Not applicable (retrospective analysis).
Consent to publish
The authors affirm that patients provided informed consent for publication of the images in Figs. 2 and 3.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banys-Paluchowski, M., Brus, L., Krawczyk, N. et al. Latissimus dorsi flap for breast reconstruction: a large single-institution evaluation of surgical outcome and complications. Arch Gynecol Obstet 309, 269–280 (2024). https://doi.org/10.1007/s00404-023-07186-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07186-3