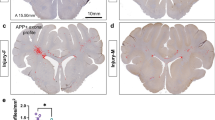
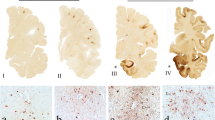
Abstract
Despite being a major health concern, little is known about the pathophysiological changes that underly concussion. Nonetheless, emerging evidence suggests that selective damage to white matter axons, or diffuse axonal injury (DAI), disrupts brain network connectivity and function. While voltage-gated sodium channels (NaChs) and their anchoring proteins at the nodes of Ranvier (NOR) on axons are key elements of the brain’s network signaling machinery, changes in their integrity have not been studied in context with DAI. Here, we utilized a clinically relevant swine model of concussion that induces evolving axonal pathology, demonstrated by accumulation of amyloid precursor protein (APP) across the white matter. Over a two-week follow-up post-concussion with this model, we found widespread loss of NaCh isoform 1.6 (Nav1.6), progressive increases in NOR length, the appearance of void and heminodes and loss of βIV-spectrin, ankyrin G, and neurofascin 186 or their collective diffusion into the paranode. Notably, these changes were in close proximity, yet distinct from APP-immunoreactive swollen axonal profiles, potentially representing a unique, newfound phenotype of axonal pathology in DAI. Since concussion in humans is non-fatal, the clinical relevance of these findings was determined through examination of post-mortem brain tissue from humans with higher levels of acute traumatic brain injury. Here, a similar loss of Nav1.6 and changes in NOR structures in brain white matter were observed as found in the swine model of concussion. Collectively, this widespread and progressive disruption of NaChs and NOR appears to be a form of sodium channelopathy, which may represent an important substrate underlying brain network dysfunction after concussion.








Similar content being viewed by others
Data and materials availability
All data are available in the main text and the supplementary materials.
References
Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR (1989) Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 15:49–59. https://doi.org/10.1111/j.1365-2559.1989.tb03040.x
Adams JH, Graham DI, Gennarelli TA, Maxwell WL (1991) Diffuse axonal injury in non-missile head injury. J Neurol Neurosurg Psychiatry 54:481–483. https://doi.org/10.1136/jnnp.54.6.481
Amor V, Feinberg K, Eshed-Eisenbach Y, Vainshtein A, Frechter S, Grumet M et al (2014) Long-term maintenance of Na+ channels at nodes of Ranvier depends on glial contact mediated by gliomedin and NrCAM. J Neurosci 34:5089–5098. https://doi.org/10.1523/JNEUROSCI.4752-13.2014
Amor V, Zhang C, Vainshtein A, Zhang A, Zollinger DR, Eshed-Eisenbach Y et al (2017) The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier. Elife. https://doi.org/10.7554/eLife.21392
Arancibia-Carcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D (2017) Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife. https://doi.org/10.7554/eLife.23329
Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM (2016) White matter involvement after TBI: clues to axon and myelin repair capacity. Exp Neurol 275(Pt 3):328–333. https://doi.org/10.1016/j.expneurol.2015.02.011
Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D (2007) Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 24:1447–1459. https://doi.org/10.1089/neu.2007.0241
Bean BP (2007) The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465. https://doi.org/10.1038/nrn2148
Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ (1994) Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 344:1055–1056. https://doi.org/10.1016/s0140-6736(94)91712-4
Broglio SP, Lapointe A, O’Connor KL, McCrea M (2017) Head impact density: a model to explain the elusive concussion threshold. J Neurotrauma 34:2675–2683. https://doi.org/10.1089/neu.2016.4767
Browne KD, Chen XH, Meaney DF, Smith DH (2011) Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma 28:1747–1755. https://doi.org/10.1089/neu.2011.1913
Catterall WA (2017) Forty years of sodium channels: structure, function, pharmacology, and epilepsy. Neurochem Res 42:2495–2504. https://doi.org/10.1007/s11064-017-2314-9
Chen L, Huang J, Zhao P, Persson AK, Dib-Hajj FB, Cheng X et al (2018) Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Sci Rep 8:3845. https://doi.org/10.1038/s41598-018-22216-w
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6:284–290. https://doi.org/10.1037/1040-3590.6.4.284
Cicchetti DV (2001) The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol 23:695–700. https://doi.org/10.1076/jcen.23.5.695.1249
Cullen DK, Harris JP, Browne KD, Wolf JA, Duda JE, Meaney DF et al (2016) A porcine model of traumatic brain injury via head rotational acceleration. Methods Mol Biol 1462:289–324. https://doi.org/10.1007/978-1-4939-3816-2_17
Duma SM, Manoogian SJ, Bussone WR, Brolinson PG, Goforth MW, Donnenwerth JJ et al (2005) Analysis of real-time head accelerations in collegiate football players. Clin J Sport Med 15:3–8. https://doi.org/10.1097/00042752-200501000-00002
Eshed-Eisenbach Y, Peles E (2013) The making of a node: a co-production of neurons and glia. Curr Opin Neurobiol 23:1049–1056. https://doi.org/10.1016/j.conb.2013.06.003
Felmingham KL, Baguley IJ, Green AM (2004) Effects of diffuse axonal injury on speed of information processing following severe traumatic brain injury. Neuropsychology 18:564–571. https://doi.org/10.1037/0894-4105.18.3.564
Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C et al (2015) Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun 6:8073. https://doi.org/10.1038/ncomms9073
Frechede B, McIntosh AS (2009) Numerical reconstruction of real-life concussive football impacts. Med Sci Sports Exerc 41:390–398. https://doi.org/10.1249/MSS.0b013e318186b1c5
Freeman SA, Desmazieres A, Fricker D, Lubetzki C, Sol-Foulon N (2016) Mechanisms of sodium channel clustering and its influence on axonal impulse conduction. Cell Mol Life Sci 73:723–735. https://doi.org/10.1007/s00018-015-2081-1
Gasser A, Ho TS, Cheng X, Chang KJ, Waxman SG, Rasband MN et al (2012) An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J Neurosci 32:7232–7243. https://doi.org/10.1523/JNEUROSCI.5434-11.2012
Graham DI, Gentleman SM, Lynch A, Roberts GW (1995) Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol 21:27–34. https://doi.org/10.1111/j.1365-2990.1995.tb01025.x
Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF et al (2004) Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 24:4605–4613. https://doi.org/10.1523/JNEUROSCI.0515-03.2004
Jenkins SM, Bennett V (2002) Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc Natl Acad Sci USA 99:2303–2308. https://doi.org/10.1073/pnas.042601799
Jette N, Coderre E, Nikolaeva MA, Enright PD, Iwata A, Smith DH et al (2006) Spatiotemporal distribution of spectrin breakdown products induced by anoxia in adult rat optic nerve in vitro. J Cereb Blood Flow Metab 26:777–786. https://doi.org/10.1038/sj.jcbfm.9600226
Johnson VE, Meaney DF, Cullen DK, Smith DH (2015) Animal models of traumatic brain injury. Handb Clin Neurol 127:115–128. https://doi.org/10.1016/B978-0-444-52892-6.00008-8
Johnson VE, Stewart W, Arena JD, Smith DH (2017) Traumatic brain injury as a trigger of neurodegeneration. Adv Neurobiol 15:383–400. https://doi.org/10.1007/978-3-319-57193-5_15
Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W (2013) Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136:28–42. https://doi.org/10.1093/brain/aws322
Johnson VE, Stewart W, Smith DH (2013) Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. https://doi.org/10.1016/j.expneurol.2012.01.013
Johnson VE, Stewart W, Weber MT, Cullen DK, Siman R, Smith DH (2016) SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol 131:115–135. https://doi.org/10.1007/s00401-015-1506-0
Johnson VE, Weber MT, Xiao R, Cullen DK, Meaney DF, Stewart W et al (2018) Mechanical disruption of the blood–brain barrier following experimental concussion. Acta Neuropathol 135:711–726. https://doi.org/10.1007/s00401-018-1824-0
Kourtidou P, McCauley SR, Bigler ED, Traipe E, Wu TC, Chu ZD et al (2013) Centrum semiovale and corpus callosum integrity in relation to information processing speed in patients with severe traumatic brain injury. J Head Trauma Rehabil 28:433–441. https://doi.org/10.1097/HTR.0b013e3182585d06
Labasque M, Devaux JJ, Leveque C, Faivre-Sarrailh C (2011) Fibronectin type III-like domains of neurofascin-186 protein mediate gliomedin binding and its clustering at the developing nodes of Ranvier. J Biol Chem 286:42426–42434. https://doi.org/10.1074/jbc.M111.266353
Leclercq PD, McKenzie JE, Graham DI, Gentleman SM (2001) Axonal injury is accentuated in the caudal corpus callosum of head-injured patients. J Neurotrauma 18:1–9. https://doi.org/10.1089/089771501750055721
Liu CH, Rasband MN (2019) Axonal spectrins: nanoscale organization, functional domains and spectrinopathies. Front Cell Neurosci 13:234. https://doi.org/10.3389/fncel.2019.00234
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A et al (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16:987–1048. https://doi.org/10.1016/S1474-4422(17)30371-X
Marion CM, Radomski KL, Cramer NP, Galdzicki Z, Armstrong RC (2018) Experimental traumatic brain injury identifies distinct early and late phase axonal conduction deficits of white matter pathophysiology, and reveals intervening recovery. J Neurosci 38:8723–8736. https://doi.org/10.1523/JNEUROSCI.0819-18.2018
Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D et al (2010) A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74:643–650. https://doi.org/10.1212/WNL.0b013e3181d0ccdd
Meaney DF, Smith DH (2011) Biomechanics of concussion. Clin Sports Med 30(19–31):vii. https://doi.org/10.1016/j.csm.2010.08.009
Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E et al (2019) Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) study. JAMA Neurol 76:1049–1059. https://doi.org/10.1001/jamaneurol.2019.1313
Ozen I, Arkan S, Clausen F, Ruscher K, Marklund N (2022) Diffuse traumatic injury in the mouse disrupts axon-myelin integrity in the cerebellum. J Neurotrauma 39:411–422. https://doi.org/10.1089/neu.2021.0321
Palacios EM, Owen JP, Yuh EL, Wang MB, Vassar MJ, Ferguson AR et al (2020) The evolution of white matter microstructural changes after mild traumatic brain injury: a longitudinal DTI and NODDI study. Sci Adv 6:eaaz6892. https://doi.org/10.1126/sciadv.aaz6892
Pfister BJ, Bonislawski DP, Smith DH, Cohen AS (2006) Stretch-grown axons retain the ability to transmit active electrical signals. FEBS Lett 580:3525–3531. https://doi.org/10.1016/j.febslet.2006.05.030
Raghavan M, Fee D, Barkhaus PE (2019) Generation and propagation of the action potential. Handb Clin Neurol 160:3–22. https://doi.org/10.1016/B978-0-444-64032-1.00001-1
Rasband MN, Peles E (2015) The nodes of Ranvier: molecular assembly and maintenance. Cold Spring Harb Perspect Biol 8:a020495. https://doi.org/10.1101/cshperspect.a020495
Rasband MN, Peles E (2021) Mechanisms of node of Ranvier assembly. Nat Rev Neurosci 22:7–20. https://doi.org/10.1038/s41583-020-00406-8
Reeves TM, Greer JE, Vanderveer AS, Phillips LL (2010) Proteolysis of submembrane cytoskeletal proteins ankyrin-G and alphaII-spectrin following diffuse brain injury: a role in white matter vulnerability at nodes of Ranvier. Brain Pathol 20:1055–1068. https://doi.org/10.1111/j.1750-3639.2010.00412.x
Reeves TM, Phillips LL, Povlishock JT (2005) Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 196:126–137. https://doi.org/10.1016/j.expneurol.2005.07.014
von Reyn CR, Mott RE, Siman R, Smith DH, Meaney DF (2012) Mechanisms of calpain mediated proteolysis of voltage gated sodium channel alpha-subunits following in vitro dynamic stretch injury. J Neurochem 121:793–805. https://doi.org/10.1111/j.1471-4159.2012.07735.x
von Reyn CR, Spaethling JM, Mesfin MN, Ma M, Neumar RW, Smith DH et al (2009) Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J Neurosci 29:10350–10356. https://doi.org/10.1523/JNEUROSCI.2339-09.2009
Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J et al (2000) Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci 20:8354–8364
Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J et al (2003) Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci 23:7001–7011
Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H (2008) Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 100:2361–2380. https://doi.org/10.1152/jn.90332.2008
Rush AM, Wittmack EK, Tyrrell L, Black JA, Dib-Hajj SD, Waxman SG (2006) Differential modulation of sodium channel Na(v)1.6 by two members of the fibroblast growth factor homologous factor 2 subfamily. Eur J Neurosci 23:2551–2562. https://doi.org/10.1111/j.1460-9568.2006.04789.x
Shahim P, Tegner Y, Wilson DH, Randall J, Skillback T, Pazooki D et al (2014) Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 71:684–692. https://doi.org/10.1001/jamaneurol.2014.367
Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA et al (2005) Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48:737–742. https://doi.org/10.1016/j.neuron.2005.10.019
Siman R, Shahim P, Tegner Y, Blennow K, Zetterberg H, Smith DH (2015) Serum SNTF increases in concussed professional ice hockey players and relates to the severity of postconcussion symptoms. J Neurotrauma 32:1294–1300. https://doi.org/10.1089/neu.2014.3698
Smith DH, Dolle JP, Ameen-Ali KE, Bretzin A, Cortes E, Crary JF et al (2021) Collaborative neuropathology network characterizing outcomes of TBI (CONNECT-TBI). Acta Neuropathol Commun 9:32. https://doi.org/10.1186/s40478-021-01122-9
Smith DH, Johnson VE, Trojanowski JQ, Stewart W (2019) Chronic traumatic encephalopathy—confusion and controversies. Nat Rev Neurol 15:179–183. https://doi.org/10.1038/s41582-018-0114-8
Smith DH, Nonaka M, Miller R, Leoni M, Chen XH, Alsop D et al (2000) Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J Neurosurg 93:315–322. https://doi.org/10.3171/jns.2000.93.2.0315
Smith DH, Stewart W (2020) “Concussion” is not a true diagnosis. Nat Rev Neurol 16:457–458. https://doi.org/10.1038/s41582-020-0382-y
Song H, Chen C, Kelley B, Tomasevich A, Lee H, Dolle JP et al (2022) Traumatic brain injury recapitulates developmental changes of axons. Prog Neurobiol 217:102332. https://doi.org/10.1016/j.pneurobio.2022.102332
Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L et al (2000) An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J Cell Biol 150:657–666. https://doi.org/10.1083/jcb.150.3.657
Ueda R, Hara H, Hata J, Senoo A (2021) White matter degeneration in diffuse axonal injury and mild traumatic brain injury observed with automatic tractography. Neuroreport 32:936–941. https://doi.org/10.1097/WNR.0000000000001688
Wolf JA, Johnson BN, Johnson VE, Putt ME, Browne KD, Mietus CJ et al (2017) Concussion induces hippocampal circuitry disruption in swine. J Neurotrauma 34:2303–2314. https://doi.org/10.1089/neu.2016.4848
Yallampalli R, Wilde EA, Bigler ED, McCauley SR, Hanten G, Troyanskaya M et al (2013) Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J Neuroimaging 23:224–227. https://doi.org/10.1111/j.1552-6569.2010.00537.x
Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN (2004) BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci 24:7230–7240. https://doi.org/10.1523/JNEUROSCI.2125-04.2004
Yuen TJ, Browne KD, Iwata A, Smith DH (2009) Sodium channelopathy induced by mild axonal trauma worsens outcome after a repeat injury. J Neurosci Res 87:3620–3625. https://doi.org/10.1002/jnr.22161
Zhou W, Goldin AL (2004) Use-dependent potentiation of the Nav1.6 sodium channel. Biophys J 87:3862–3872. https://doi.org/10.1529/biophysj.104.045963
Acknowledgements
Here, we thank Drs. Stephen Waxman, Sulayman Dib-Hajj, and Shujun Liu from Yale University, Dr. Matthew Rasband from Baylor College of Medicine, and Dr. Peter Brophy from The University of Edinburgh for providing primary antibodies used in this study. We also thank Dr. David F. Meaney for using the Leica SP5 confocal microscope, and Cell & Developmental Biology (CDB) microscopy core at University of Pennsylvania for using the Zeiss LSM 880 with Airyscan confocal microscope and Imaris software (GraphPad, Bitplane, v.9.7) with the help from Drs. Andrea Stout, Xinyu Zhao, and Matthew J. Gastinger for the initial setup. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This research was made available with the following support from National Institutes of Health grants R01NS092398 (DHS), R01NS038104 (DHS), R01NS094003 (DHS), R01EB021293 (DHS), U54NS115322 (WS and DHS), as well as support from Paul G. Allen Family Foundation (DHS) and the Pennsylvania Department of Health Consortium on Traumatic Brain Injury 4100077083 (DHS).
Author information
Authors and Affiliations
Contributions
Each author’s contributions to the paper are listed. Conceptualization: HS, PPM, DHS; Methodology: HS, PPM, KEAA, AT, CKD, AP, EJA, VEJ, WS, DHS; Investigation: HS, PPM, KEAA, WS, DHS; Visualization: HS; Funding acquisition: WS, DHS; Supervision: HS, VEJ, JPD, WS, DHS; Writing—original draft: HS, DHS; Writing—review & editing: HS, JPD, WS, DHS.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H., McEwan, P.P., Ameen-Ali, K.E. et al. Concussion leads to widespread axonal sodium channel loss and disruption of the node of Ranvier. Acta Neuropathol 144, 967–985 (2022). https://doi.org/10.1007/s00401-022-02498-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02498-1