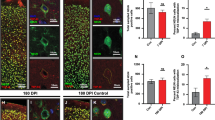
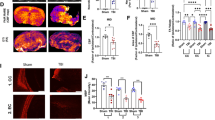
Abstract
Traumatic brain injury (TBI) has been recognized as an important risk factor for Alzheimer’s disease (AD). However, the molecular mechanisms by which TBI contributes to developing AD remain unclear. Here, we provide evidence that aberrant production of TDP-43 is a key factor in promoting AD neuropathology and synaptic and cognitive deterioration in mouse models of mild closed head injury (CHI). We observed that a single mild CHI is sufficient to exacerbate AD neuropathology and accelerate synaptic and cognitive deterioration in APP transgenic mice but repeated mild CHI are required to induce neuropathological changes and impairments in synaptic plasticity, spatial learning, and memory retention in wild-type animals. Importantly, these changes in animals exposed to a single or repeated mild CHI are alleviated by silencing of TDP-43 but reverted by rescue of the TDP-43 knockdown. Moreover, overexpression of TDP-43 in the hippocampus aggravates AD neuropathology and provokes cognitive impairment in APP transgenic mice, mimicking single mild CHI-induced changes. We further discovered that neuroinflammation triggered by TBI promotes NF-κB-mediated transcription and expression of TDP-43, which in turn stimulates tau phosphorylation and Aβ formation. Our findings suggest that excessive production of TDP-43 plays an important role in exacerbating AD neuropathology and in driving synaptic and cognitive declines following TBI.











Similar content being viewed by others
Data availability
Data supporting the findings of this manuscript are available the main text and the supplementary materials or from the corresponding authors upon request.
References
Al-Dahhak R, Khoury R, Qazi E, Grossberg GT (2018) Traumatic brain injury, chronic traumatic encephalopathy, and Alzheimer disease. Clin Geriatr Med 34(4):617–635. https://doi.org/10.1016/j.cger.2018.06.008
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110. https://doi.org/10.1007/s10339-011-0430-z
Arulsamy A, Corrigan F, Collins-Praino LE (2019) Cognitive and neuropsychiatric impairments vary as a function of injury severity at 12 months post-experimental diffuse traumatic brain injury: implications for dementia development. Behav Brain Res. https://doi.org/10.1016/j.bbr.2019.02.045
Blennow K, Brody DL, Kochanek PM, Levin H, McKee A et al (2016) Traumatic brain injuries. Nat Rev Dis Primers. https://doi.org/10.1038/nrdp.2016.84
Blennow K, Hardy J, Zetterberg H (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76(5):886–899. https://doi.org/10.1016/j.neuron.2012.11.021
Blokhuis AM, Koppers M, Groen EJN, van den Heuvel DMA, Dini Modigliani S et al (2016) Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways. Acta Neuropathol 132(2):175–196. https://doi.org/10.1007/s00401-016-1575-8
Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71(4):505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Boeynaems S, Bogaert E, Van Damme P, Van Den Bosch L (2016) Inside out: the role of nucleocytoplasmic transport in ALS and FTLD. Acta Neuropathol 132(2):159–173. https://doi.org/10.1007/s00401-016-1586-5
Breunig JJ, Guillot-Sestier MV, Town T (2013) Brain injury, neuroinflammation and Alzheimer’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2013.00026
Budini M, Baralle FE, Buratti E (2014) Targeting TDP-43 in neurodegenerative diseases. Expert Opin Ther Targets 18(6):617–632. https://doi.org/10.1517/14728222.2014.896905
Busche MA, Hyman BT (2020) Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci 23(10):1183–1193. https://doi.org/10.1038/s41593-020-0687-6
Chen R, Zhang J, Fan N, Teng ZQ, Wu Y et al (2013) Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 155(5):1154–1165. https://doi.org/10.1016/j.cell.2013.10.042
Chen R, Zhang J, Wu Y, Wang D, Feng G et al (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep 2(5):1329–1339. https://doi.org/10.1016/j.celrep.2012.09.030
Cheng JS, Craft R, Yu GQ, Ho K, Wang X et al (2014) Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS ONE 9(12):e115765. https://doi.org/10.1371/journal.pone.0115765
Cherry JD, Esnault CD, Baucom ZH, Tripodis Y, Huber BR et al (2021) Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer’s disease. Acta Neuropathol Commun 9(1):86. https://doi.org/10.1186/s40478-021-01189-4
Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I et al (2018) TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci 21(2):228–239. https://doi.org/10.1038/s41593-017-0047-3
Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP et al (2011) The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. https://doi.org/10.1038/ncomms1255
Dams-O’Connor K, Guetta G, Hahn-Ketter AE, Fedor A (2016) Traumatic brain injury as a risk factor for Alzheimer’s disease: current knowledge and future directions. Neurodegener Dis Manag 6(5):417–429. https://doi.org/10.2217/nmt-2016-0017
de Boer EMJ, Orie VK, Williams T, Baker MR, De Oliveira HM et al (2020) TDP-43 proteinopathies: a new wave of neurodegenerative diseases. J Neurol Neurosurg Psychiatry 92(1):86–95. https://doi.org/10.1136/jnnp-2020-322983
Delic V, Beck KD, Pang KCH, Citron BA (2020) Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol Commun 8(1):45. https://doi.org/10.1186/s40478-020-00924-7
Djordjevic J, Sabbir MG, Albensi BC (2016) Traumatic brain injury as a risk factor for Alzheimer’s disease: is inflammatory signaling a key player? Curr Alzheimer Res 13(7):730–738
Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74(7):857–862
Gao J, Wang L, Huntley ML, Perry G, Wang X (2018) Pathomechanisms of TDP-43 in neurodegeneration. J Neurochem. https://doi.org/10.1111/jnc.14327
Gardner RC, Yaffe K (2015) Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 66(Pt B):75–80. https://doi.org/10.1016/j.mcn.2015.03.001
Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW (1993) Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett 160(2):139–144. https://doi.org/10.1016/0304-3940(93)90398-5
Gilbert M, Snyder C, Corcoran C, Norton MC, Lyketsos CG et al (2014) The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study. Int Psychogeriatr 26(10):1593–1601. https://doi.org/10.1017/s1041610214000842
Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L et al (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54(6):1316–1323
Gupta R, Sen N (2016) Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev Neurosci 27(1):93–100. https://doi.org/10.1515/revneuro-2015-0017
Hashem J, Hu M, Zhang J, Gao F, Chen C (2021) Inhibition of 2-arachidonoylglycerol metabolism alleviates neuropathology and improves cognitive function in a Tau mouse model of Alzheimer’s disease. Mol Neurobiol 58(8):4122–4133. https://doi.org/10.1007/s12035-021-02400-2
Heyburn L, Sajja V, Long JB (2019) The role of TDP-43 in military-relevant TBI and chronic neurodegeneration. Front Neurol. https://doi.org/10.3389/fneur.2019.00680
Hu M, Zhu D, Zhang J, Gao F, Hashem J et al (2022) Enhancing endocannabinoid signalling in astrocytes promotes recovery from traumatic brain injury. Brain 145(1):179–193. https://doi.org/10.1093/brain/awab310
Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX et al (2012) Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain 135(Pt 3):807–818. https://doi.org/10.1093/brain/aws013
Janssens J, Van Broeckhoven C (2013) Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Hum Mol Genet 22(R1):R77-87. https://doi.org/10.1093/hmg/ddt349
Johnson VE, Stewart W, Arena JD, Smith DH (2017) Traumatic brain injury as a trigger of neurodegeneration. Adv Neurobiol. https://doi.org/10.1007/978-3-319-57193-5_15
Johnson VE, Stewart W, Smith DH (2010) Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci 11(5):361–370. https://doi.org/10.1038/nrn2808
Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME et al (2015) TDP-43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. https://doi.org/10.1002/ana.24493
Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N et al (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127(6):811–824. https://doi.org/10.1007/s00401-014-1269-z
Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40(5):572–574. https://doi.org/10.1038/ng.132
Klim JR, Pintacuda G, Nash LA, Guerra San Juan I, Eggan K (2021) Connecting TDP-43 Pathology with Neuropathy. Trends Neurosci 44(6):424–440. https://doi.org/10.1016/j.tins.2021.02.008
Kraemer BC, Schuck T, Wheeler JM, Robinson LC, Trojanowski JQ et al (2010) Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol 119(4):409–419. https://doi.org/10.1007/s00401-010-0659-0
Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, EURODEM Incidence Research Group and Work Groups. European Studies of Dementia et al (1999) Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology 52(1):78–84. https://doi.org/10.1212/wnl.52.1.78
Lauretti E, Dincer O (1867) Praticò D (2020) Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim Biophys Acta Mol Cell Res 5:118664. https://doi.org/10.1016/j.bbamcr.2020.118664
Lecca D, Bader M, Tweedie D, Hoffman AF, Jung YJ et al (2019) (-)-Phenserine and the prevention of pre-programmed cell death and neuroinflammation in mild traumatic brain injury and Alzheimer’s disease challenged mice. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2019.104528
Li Y, Li Y, Li X, Zhang S, Zhao J et al (2017) Head injury as a risk factor for dementia and Alzheimer’s disease: a systematic review and meta-analysis of 32 observational studies. PLoS ONE 12(1):e0169650. https://doi.org/10.1371/journal.pone.0169650
Ling SC, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79(3):416–438. https://doi.org/10.1016/j.neuron.2013.07.033
Llorens-Martin M, Jurado J, Hernandez F, Avila J (2014) GSK-3beta, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2014.00046
LoBue C, Munro C, Schaffert J, Didehbani N, Hart J et al (2019) Traumatic brain injury and risk of long-term brain changes, accumulation of pathological markers, and developing dementia: a review. J Alzheimers Dis 70(3):629–654. https://doi.org/10.3233/jad-190028
Luo J, Nguyen A, Villeda S, Zhang H, Ding Z et al (2014) Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol. https://doi.org/10.3389/fneur.2014.00012
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG et al (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27(4):472–479. https://doi.org/10.1111/bpa.12424
Medina M, Garrido JJ, Wandosell FG (2011) Modulation of GSK-3 as a therapeutic strategy on Tau pathologies. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2011.00024
Meehan WP 3rd, Zhang J, Mannix R, Whalen MJ (2012) Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71(4):885–891. https://doi.org/10.1227/NEU.0b013e318265a439
Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM et al (1999) Head trauma and risk of dementia and Alzheimer’s disease: the Rotterdam study. Neurology 53(9):1959–1962. https://doi.org/10.1212/wnl.53.9.1959
Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V et al (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67(6):953–966. https://doi.org/10.1016/j.neuron.2010.08.044
Mortimer JA, French LR, Hutton JT, Schuman LM (1985) Head injury as a risk factor for Alzheimer’s disease. Neurology 35(2):264–267
Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA et al (2018) TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 6(1):33. https://doi.org/10.1186/s40478-018-0531-3
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. https://doi.org/10.1126/science.1134108
Oakley H, Cole SL, Logan S, Maus E, Shao P et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26(40):10129–10140. https://doi.org/10.1523/jneurosci.1202-06.2006
Petraglia AL, Dashnaw ML, Turner RC, Bailes JE (2014) Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery 75(4):S34-49. https://doi.org/10.1227/neu.0000000000000472
Petraglia AL, Plog BA, Dayawansa S, Dashnaw ML, Czerniecka K et al (2014) The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int. https://doi.org/10.4103/2152-7806.147566
Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel BK (2019) Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2019.00025
Sephton CF, Good SK, Atkin S, Dewey CM, Mayer P 3rd et al (2010) TDP-43 is a developmentally regulated protein essential for early embryonic development. J Biol Chem 285(9):6826–6834. https://doi.org/10.1074/jbc.M109.061846
Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K et al (2021) Reducing acetylated tau is neuroprotective in brain injury. Cell. https://doi.org/10.1016/j.cell.2021.03.032
Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA et al (2011) Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70(7):551–567. https://doi.org/10.1097/NEN.0b013e31821f891f
Sivanandam TM, Thakur MK (2012) Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev 36(5):1376–1381. https://doi.org/10.1016/j.neubiorev.2012.02.013
Smith DH, Johnson VE, Trojanowski JQ, Stewart W (2019) Chronic traumatic encephalopathy—confusion and controversies. Nat Rev Neurol 15(3):179–183. https://doi.org/10.1038/s41582-018-0114-8
Song Y, Hu M, Zhang J, Teng ZQ, Chen C (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine. https://doi.org/10.1016/j.ebiom.2018.11.059
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319(5870):1668–1672. https://doi.org/10.1126/science.1154584
Sussman ES, Pendharkar AV, Ho AL, Ghajar J (2018) Mild traumatic brain injury and concussion: terminology and classification. Handb Clin Neurol. https://doi.org/10.1016/b978-0-444-63954-7.00003-3
Takashima A (2006) GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 9(3 Suppl):309–317. https://doi.org/10.3233/jad-2006-9s335
Tsitsopoulos PP, Marklund N (2013) Amyloid-beta peptides and Tau protein as biomarkers in cerebrospinal and interstitial fluid following traumatic brain injury: a review of experimental and clinical studies. Front Neurol. https://doi.org/10.3389/fneur.2013.00079
Turner RC, Lucke-Wold BP, Robson MJ, Lee JM, Bailes JE (2016) Alzheimer’s disease and chronic traumatic encephalopathy: distinct but possibly overlapping disease entities. Brain Inj 30(11):1279–1292. https://doi.org/10.1080/02699052.2016.1193631
Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2013.00029
Washington PM, Villapol S, Burns MP (2016) Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 275:381–388. https://doi.org/10.1016/j.expneurol.2015.06.015
Wilson AC, Dugger BN, Dickson DW, Wang DS (2011) TDP-43 in aging and Alzheimer’s disease—a review. Int J Clin Exp Pathol 4(2):147–155
Wu Y, Wu H, Zeng J, Pluimer B, Dong S et al (2021) Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol Commun 9(1):74. https://doi.org/10.1186/s40478-021-01178-7
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N et al (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53(3):337–351. https://doi.org/10.1016/j.neuron.2007.01.010
Zhang J, Chen C (2018) Alleviation of neuropathology by inhibition of monoacylglycerol lipase in APP transgenic mice lacking CB2 receptors. Mol Neurobiol 55(6):4802–4810. https://doi.org/10.1007/s12035-017-0689-x
Zhang J, Chen C (2008) Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem 283(33):22601–22611. https://doi.org/10.1074/jbc.M800524200
Zhang J, Hu M, Teng Z, Tang YP, Chen C (2014) Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J Neurosci 34(45):14919–14933. https://doi.org/10.1523/jneurosci.1165-14.2014
Zhang J, Teng Z, Song Y, Hu M, Chen C (2015) Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab 35(3):443–453. https://doi.org/10.1038/jcbfm.2014.216
Acknowledgements
We thank Dr. Bryan W. Luikart of Dartmouth Medical School for providing FUGW lentiviral vectors and Dr. Zhao-qian Teng for participating in the initial work of this project. This work was supported by National Institutes of Health grants R01NS076815, R01MH113535, and R01AG058621 (to C.C.) and by startup funds from UT Health San Antonio, Joe R. & Teresa Lozano Long School of Medicine (to C.C.).
Author information
Authors and Affiliations
Contributions
JZ and CC: conceived the project and designed the experiments; JZ, MH, FG and JH: performed the experiments and analyzed the data; CC: supervised the work and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, F., Hu, M., Zhang, J. et al. TDP-43 drives synaptic and cognitive deterioration following traumatic brain injury. Acta Neuropathol 144, 187–210 (2022). https://doi.org/10.1007/s00401-022-02449-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02449-w