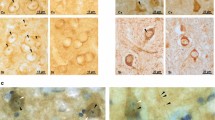
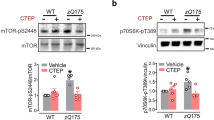
Abstract
Impaired neuronal proteostasis is a salient feature of many neurodegenerative diseases, highlighting alterations in the function of the endoplasmic reticulum (ER). We previously reported that targeting the transcription factor XBP1, a key mediator of the ER stress response, delays disease progression and reduces protein aggregation in various models of neurodegeneration. To identify disease modifier genes that may explain the neuroprotective effects of XBP1 deficiency, we performed gene expression profiling of brain cortex and striatum of these animals and uncovered insulin-like growth factor 2 (Igf2) as the major upregulated gene. Here, we studied the impact of IGF2 signaling on protein aggregation in models of Huntington’s disease (HD) as proof of concept. Cell culture studies revealed that IGF2 treatment decreases the load of intracellular aggregates of mutant huntingtin and a polyglutamine peptide. These results were validated using induced pluripotent stem cells (iPSC)-derived medium spiny neurons from HD patients and spinocerebellar ataxia cases. The reduction in the levels of mutant huntingtin was associated with a decrease in the half-life of the intracellular protein. The decrease in the levels of abnormal protein aggregation triggered by IGF2 was independent of the activity of autophagy and the proteasome pathways, the two main routes for mutant huntingtin clearance. Conversely, IGF2 signaling enhanced the secretion of soluble mutant huntingtin species through exosomes and microvesicles involving changes in actin dynamics. Administration of IGF2 into the brain of HD mice using gene therapy led to a significant decrease in the levels of mutant huntingtin in three different animal models. Moreover, analysis of human postmortem brain tissue and blood samples from HD patients showed a reduction in IGF2 level. This study identifies IGF2 as a relevant factor deregulated in HD, operating as a disease modifier that buffers the accumulation of abnormal protein species.









Similar content being viewed by others
References
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C et al (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27:53–66. https://doi.org/10.1016/j.molcel.2007.06.011
Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S et al (2011) A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J 30:4071–4083. https://doi.org/10.1038/emboj.2011.293
Alberini CM, Chen DY (2012) Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci 35:274–283. https://doi.org/10.1016/j.tins.2011.12.007
Allodi I, Comley L, Nichterwitz S, Nizzardo M, Simone C, Benitez JA et al (2016) Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci Rep 6:25960. https://doi.org/10.1038/srep25960
Babcock DT, Ganetzky B (2015) Transcellular spreading of huntingtin aggregates in the Drosophila brain. Proc Natl Acad Sci USA 112:E5427–5433. https://doi.org/10.1073/pnas.1516217112
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319:916–919. https://doi.org/10.1126/science.1141448
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR et al (2015) Huntington disease. Nat Rev Dis Primers 1:15005. https://doi.org/10.1038/nrdp.2015.5
Bergman D, Halje M, Nordin M, Engstrom W (2013) Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 59:240–249. https://doi.org/10.1159/000343995
Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115:1449–1457. https://doi.org/10.1172/JCI24761
Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J et al (2012) Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci 32:3376–3387. https://doi.org/10.1523/JNEUROSCI.4248-11.2012
Caroni P, Grandes P (1990) Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol 110:1307–1317. https://doi.org/10.1083/jcb.110.4.1307
Chamberlain SJ (2016) Disease modelling using human iPSCs. Hum Mol Genet 25:R173–R181. https://doi.org/10.1093/hmg/ddw209
Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D et al (2011) A critical role for IGF-II in memory consolidation and enhancement. Nature 469:491–497. https://doi.org/10.1038/nature09667
Chiti F, Dobson CM (2017) Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86:27–68. https://doi.org/10.1146/annurev-biochem-061516-045115
Cicchetti F, Saporta S, Hauser RA, Parent M, Saint-Pierre M, Sanberg PR et al (2009) Neural transplants in patients with Huntington's disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci USA 106:12483–12488. https://doi.org/10.1073/pnas.0904239106
DeChiara TM, Robertson EJ, Efstratiadis A (1991) Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64:849–859. https://doi.org/10.1016/0092-8674(91)90513-x
Di Prospero NA, Fischbeck KH (2005) Therapeutics development for triplet repeat expansion diseases. Nat Rev Genet 6:756–765. https://doi.org/10.1038/nrg1690
Dickson DW, Weller RO (2011) Neurodegeneration: the molecular pathology of dementia and movement disorders. https://doi.org/10.1002/9781444341256
Duran-Aniotz C, Cornejo VH, Espinoza S, Ardiles AO, Medinas DB, Salazar C et al (2017) IRE1 signaling exacerbates Alzheimer's disease pathogenesis. Acta Neuropathol 134:489–506. https://doi.org/10.1007/s00401-017-1694-x
Eijssen LM, Goelela VS, Kelder T, Adriaens ME, Evelo CT, Radonjic M (2015) A user-friendly workflow for analysis of Illumina gene expression bead array data available at the arrayanalysisorg portal. BMC Genom 16:482. https://doi.org/10.1186/s12864-015-1689-8
Engstrom W, Shokrai A, Otte K, Granerus M, Gessbo A, Bierke P et al (1998) Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Prolif 31:173–189. https://doi.org/10.1111/j.1365-2184.1998.tb01196.x
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635. https://doi.org/10.1038/nature01148
Feil R, Walter J, Allen ND, Reik W (1994) Developmental control of allelic methylation in the imprinted mouse Igf2 and H19 genes. Development 120:2933–2943
Fernandez AM, Torres-Aleman I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13:225–239. https://doi.org/10.1038/nrn3209
Ferron SR, Radford EJ, Domingo-Muelas A, Kleine I, Ramme A, Gray D et al (2015) Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis. Nat Commun 6:8265. https://doi.org/10.1038/ncomms9265
Garcia-Huerta P, Troncoso-Escudero P, Jerez C, Hetz C, Vidal RL (2016) The intersection between growth factors, autophagy and ER stress: a new target to treat neurodegenerative diseases? Brain Res 1649:173–180. https://doi.org/10.1016/j.brainres.2016.02.052
Graham RK, Deng Y, Slow EJ, Haigh B, Bissada N, Lu G et al (2006) Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell 125:1179–1191. https://doi.org/10.1016/j.cell.2006.04.026
Graham RK, Slow EJ, Deng Y, Bissada N, Lu G, Pearson J et al (2006) Levels of mutant huntingtin influence the phenotypic severity of Huntington disease in YAC128 mouse models. Neurobiol Dis 21:444–455. https://doi.org/10.1016/j.nbd.2005.08.007
Hawkes C, Jhamandas JH, Harris KH, Fu W, MacDonald RG, Kar S (2006) Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein-sensitive, protein kinase C-dependent pathway. J Neurosci 26:585–596. https://doi.org/10.1523/JNEUROSCI.2730-05.2006
Hawkes C, Kar S (2004) The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res Brain Res Rev 44:117–140. https://doi.org/10.1016/j.brainresrev.2003.11.002
Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M et al (2010) Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci USA 107:9730–9735. https://doi.org/10.1073/pnas.1002575107
Henriquez DR, Bodaleo FJ, Montenegro-Venegas C, Gonzalez-Billault C (2012) The light chain 1 subunit of the microtubule-associated protein 1B (MAP1B) is responsible for Tiam1 binding and Rac1 activation in neuronal cells. PLoS ONE 7:e53123. https://doi.org/10.1371/journal.pone.0053123
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. https://doi.org/10.1038/nrm3270
Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C et al (2008) Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci USA 105:757–762. https://doi.org/10.1073/pnas.0711094105
Hetz C, Papa FR (2018) The unfolded protein response and cell fate control. Mol Cell 69:169–181. https://doi.org/10.1016/j.molcel.2017.06.017
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13:477–491. https://doi.org/10.1038/nrneurol.2017.99
Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R et al (2009) XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 23:2294–2306. https://doi.org/10.1101/gad.1830709
Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J et al (2010) Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res 16:2999–3010. https://doi.org/10.1158/1078-0432.CCR-09-3233
Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J et al (2016) Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol 132:577–592. https://doi.org/10.1007/s00401-016-1582-9
Kaushik S, Cuervo AM (2015) Proteostasis and aging. Nat Med 21:1406–1415. https://doi.org/10.1038/nm.4001
Kemp PJ, Rushton DJ, Yarova PL, Schnell C, Geater C, Hancock JM et al (2016) Improving and accelerating the differentiation and functional maturation of human stem cell-derived neurons: role of extracellular calcium and GABA. J Physiol 594:6583–6594. https://doi.org/10.1113/JP270655
Kessler SM, Haybaeck J, Kiemer AK (2016) Insulin-like growth factor 2—the oncogene and its accomplices. Curr Pharm Des 22:5948–5961. https://doi.org/10.2174/1381612822666160713100235
Khomtchouk BB, Van Booven DJ, Wahlestedt C (2014) HeatmapGenerator: high performance RNAseq and microarray visualization software suite to examine differential gene expression levels using an R and C++ hybrid computational pipeline. Source Code Biol Med 9:30. https://doi.org/10.1186/s13029-014-0030-2
Kitraki E, Bozas E, Philippidis H, Stylianopoulou F (1993) Aging-related changes in IGF-II and c-fos gene expression in the rat brain. Int J Dev Neurosci 11:1–9. https://doi.org/10.1016/0736-5748(93)90029-d
Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D et al (2011) Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature 480:543–546. https://doi.org/10.1038/nature10671
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. https://doi.org/10.1128/mcb.23.21.7448-7459.2003
Li R, Pourpak A, Morris SW (2009) Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem 52:4981–5004. https://doi.org/10.1021/jm9002395
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C et al (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506. https://doi.org/10.1016/s0092-8674(00)81369-0
Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C (2017) Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16:615–623. https://doi.org/10.1111/acel.12599
McLoughlin HS, Moore LR, Paulson HL (2020) Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol Dis 134:104635. https://doi.org/10.1016/j.nbd.2019.104635
Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC et al (2017) C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542:367–371. https://doi.org/10.1038/nature21362
Mellott TJ, Pender SM, Burke RM, Langley EA, Blusztajn JK (2014) IGF2 ameliorates amyloidosis, increases cholinergic marker expression and raises BMP9 and neurotrophin levels in the hippocampus of the APPswePS1dE9 Alzheimer's disease model mice. PLoS ONE 9:e94287. https://doi.org/10.1371/journal.pone.0094287
Mikaelsson MA, Constancia M, Dent CL, Wilkinson LS, Humby T (2013) Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat Commun 4:2311. https://doi.org/10.1038/ncomms3311
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326. https://doi.org/10.1016/j.cell.2010.01.028
Naphade S, Tshilenge KT, Ellerby LM (2019) Modeling polyglutamine expansion diseases with induced pluripotent stem cells. Neurotherapeutics 16:979–998. https://doi.org/10.1007/s13311-019-00810-8
Near SL, Whalen LR, Miller JA, Ishii DN (1992) Insulin-like growth factor II stimulates motor nerve regeneration. Proc Natl Acad Sci USA 89:11716–11720. https://doi.org/10.1073/pnas.89.24.11716
O'Riordan CR, Lachapelle AL, Vincent KA, Wadsworth SC (2000) Scaleable chromatographic purification process for recombinant adeno-associated virus (rAAV). J Gene Med 2:444–454. https://doi.org/10.1002/1521-2254(200011/12)2:6<444:AID-JGM132>3.0.CO;2-1
Ortega Z, Lucas JJ (2014) Ubiquitin-proteasome system involvement in Huntington's disease. Front Mol Neurosci 7:77. https://doi.org/10.3389/fnmol.2014.00077
Ouchi Y, Banno Y, Shimizu Y, Ando S, Hasegawa H, Adachi K et al (2013) Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci 33:9408–9419. https://doi.org/10.1523/JNEUROSCI.2700-12.2013
Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, Gonzalez-Aseguinolaza G, Mulle C, et al (2014) Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol Med 6:1246–1262. https://doi.org/10.15252/emmm.201404228
Passini MA, Wolfe JH (2001) Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol 75:12382–12392. https://doi.org/10.1128/JVI.75.24.12382-12392.2001
Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P et al (2014) Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci 17:1064–1072. https://doi.org/10.1038/nn.3761
Plate L, Cooley CB, Chen JJ, Paxman RJ, Gallagher CM, Madoux F et al (2016) Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife. https://doi.org/10.7554/eLife.15550
Porat-Shliom N, Milberg O, Masedunskas A, Weigert R (2013) Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci 70:2099–2121. https://doi.org/10.1007/s00018-012-1156-5
Pouladi MA, Xie Y, Skotte NH, Ehrnhoefer DE, Graham RK, Kim JE et al (2010) Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression. Hum Mol Genet 19:1528–1538. https://doi.org/10.1093/hmg/ddq026
Pravtcheva DD, Wise TL (2008) Igf2r improves the survival and transmission ratio of Igf2 transgenic mice. Mol Reprod Dev 75:1678–1687. https://doi.org/10.1002/mrd.20909
Proenca CC, Stoehr N, Bernhard M, Seger S, Genoud C, Roscic A et al (2013) Atg4b-dependent autophagic flux alleviates Huntington's disease progression. PLoS ONE 8:e68357. https://doi.org/10.1371/journal.pone.0068357
Ridley AJ (2001) Rho family proteins: coordinating cell responses. Trends Cell Biol 11:471–477. https://doi.org/10.1016/s0962-8924(01)02153-5
Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M et al (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5:605–607. https://doi.org/10.1038/nmeth.1220
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis 8:247–268. https://doi.org/10.3233/jad-2005-8304
Ryno LM, Genereux JC, Naito T, Morimoto RI, Powers ET, Shoulders MD et al (2014) Characterizing the altered cellular proteome induced by the stress-independent activation of heat shock factor 1. ACS Chem Biol 9:1273–1283. https://doi.org/10.1021/cb500062n
Safra M, Ben-Hamo S, Kenyon C, Henis-Korenblit S (2013) The ire-1 ER stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J Cell Sci 126:4136–4146. https://doi.org/10.1242/jcs.123000
Saleh N, Moutereau S, Azulay JP, Verny C, Simonin C, Tranchant C et al (2010) High insulinlike growth factor I is associated with cognitive decline in Huntington disease. Neurology 75:57–63. https://doi.org/10.1212/WNL.0b013e3181e62076
Salem L, Saleh N, Desamericq G, Youssov K, Dolbeau G, Cleret L et al (2016) Insulin-like growth factor-1 but not insulin predicts cognitive decline in Huntington's Disease. PLoS ONE 11:e0162890. https://doi.org/10.1371/journal.pone.0162890
Sarkar S, Rubinsztein DC (2008) Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS J 275:4263–4270. https://doi.org/10.1111/j.1742-4658.2008.06562.x
Scheper W, Hoozemans JJ (2015) The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol 130:315–331. https://doi.org/10.1007/s00401-015-1462-8
Schmeisser MJ, Baumann B, Johannsen S, Vindedal GF, Jensen V, Hvalby OC et al (2012) IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci 32:5688–5703. https://doi.org/10.1523/JNEUROSCI.0111-12.2012
Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C et al (2013) Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep 3:1279–1292. https://doi.org/10.1016/j.celrep.2013.03.024
Silva D, Dikkes P, Barnes M, Lopez MF (2009) Decreased motoneuron survival in Igf2 null mice after sciatic nerve transection. NeuroReport 20:1414–1418. https://doi.org/10.1097/WNR.0b013e328330b735
Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y et al (2003) Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet 12:1555–1567. https://doi.org/10.1093/hmg/ddg169
Smith HL, Mallucci GR (2016) The unfolded protein response: mechanisms and therapy of neurodegeneration. Brain 139:2113–2121. https://doi.org/10.1093/brain/aww101
Soares Martins T, Catita J, Martins Rosa I, Abdces O, Henriques AG (2018) Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 13:e0198820. https://doi.org/10.1371/journal.pone.0198820
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21:1332–1340. https://doi.org/10.1038/s41593-018-0235-9
Steinmetz AB, Stern SA, Kohtz AS, Descalzi G, Alberini CM (2018) Insulin-like growth factor II targets the mTOR pathway to reverse autism-like phenotypes in Mice. J Neurosci 38:1015–1029. https://doi.org/10.1523/JNEUROSCI.2010-17.2017
Stern SA, Chen DY, Alberini CM (2014) The effect of insulin and insulin-like growth factors on hippocampus- and amygdala-dependent long-term memory formation. Learn Mem 21:556–563. https://doi.org/10.1101/lm.029348.112
Suh HS, Zhao ML, Derico L, Choi N, Lee SC (2013) Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation 10:37. https://doi.org/10.1186/1742-2094-10-37
Taylor RC, Dillin A (2013) XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153:1435–1447. https://doi.org/10.1016/j.cell.2013.05.042
Thiruvalluvan A, P. de Mattos E, Brunsting JF, Bakels R, Serlidaki D, Barazzuol L et al (2020) DNAJB6, a key factor in neuronal sensitivity to amyloidogenesis. Mol Cell (in press)
Torres M, Medinas DB, Matamala JM, Woehlbier U, Cornejo VH, Solda T et al (2015) The protein-disulfide isomerase ERp57 regulates the steady-state levels of the prion protein. J Biol Chem 290:23631–23645. https://doi.org/10.1074/jbc.M114.635565
Trajkovic K, Jeong H, Krainc D (2017) Mutant Huntingtin is secreted via a late endosomal/lysosomal unconventional secretory pathway. J Neurosci 37:9000–9012. https://doi.org/10.1523/JNEUROSCI.0118-17.2017
Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC et al (2005) Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci 25:108–117. https://doi.org/10.1523/JNEUROSCI.4253-04.2005
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121. https://doi.org/10.1073/pnas.091062498
Valdes P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA et al (2014) Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci USA 111:6804–6809. https://doi.org/10.1073/pnas.1321845111
Van Raamsdonk JM, Pearson J, Rogers DA, Bissada N, Vogl AW, Hayden MR et al (2005) Loss of wild-type huntingtin influences motor dysfunction and survival in the YAC128 mouse model of Huntington disease. Hum Mol Genet 14:1379–1392. https://doi.org/10.1093/hmg/ddi147
Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR (2005) Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci 25:4169–4180. https://doi.org/10.1523/JNEUROSCI.0590-05.2005
Vidal R, Caballero B, Couve A, Hetz C (2011) Converging pathways in the occurrence of endoplasmic reticulum (ER) stress in Huntington's disease. Curr Mol Med 11:1–12. https://doi.org/10.2174/156652411794474419
Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C et al (2012) Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Hum Mol Genet 21:2245–2262. https://doi.org/10.1093/hmg/dds040
Vonsattel JP, Keller C, Cortes Ramirez EP (2011) Huntington's disease—neuropathology. Handb Clin Neurol 100:83–100. https://doi.org/10.1016/B978-0-444-52014-2.00004-5
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 44:559–577. https://doi.org/10.1097/00005072-198511000-00003
Walsh DM, Selkoe DJ (2020) Amyloid beta-protein and beyond: the path forward in Alzheimer's disease. Curr Opin Neurobiol 61:116–124. https://doi.org/10.1016/j.conb.2020.02.003
Walter HJ, Berry M, Hill DJ, Cwyfan-Hughes S, Holly JM, Logan A (1999) Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology 140:520–532. https://doi.org/10.1210/endo.140.1.6463
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. https://doi.org/10.1126/science.1209038
Wang M, Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529:326–335. https://doi.org/10.1038/nature17041
Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JR et al (2015) Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington's disease patients. J Clin Invest 125:1979–1986. https://doi.org/10.1172/JCI80743
Wise TL, Pravatcheva DD (1997) Perinatal lethality in H19 enhancers-Igf2 transgenic mice. Mol Reprod Dev 48:194–207. https://doi.org/10.1002/(SICI)1098-2795(199710)48:2<194:AID-MRD7>3.0.CO;2-N
Wong BKY, Ehrnhoefer DE, Graham RK, Martin DDO, Ladha S, Uribe V et al (2015) Partial rescue of some features of Huntington Disease in the genetic absence of caspase-6 in YAC128 mice. Neurobiol Dis 76:24–36. https://doi.org/10.1016/j.nbd.2014.12.030
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13:805–811. https://doi.org/10.1038/nn.2575
Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM et al (2015) IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med 7:272ra212. https://doi.org/10.1126/scitranslmed.3010445
Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO (2016) Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington's disease: background and evaluation in cell culture. Cell Mol Neurobiol 36:459–470. https://doi.org/10.1007/s10571-016-0350-7
Zhao T, Hong Y, Li S, Li XJ (2016) Compartment-dependent degradation of mutant Huntingtin accounts for its preferential accumulation in neuronal processes. J Neurosci 36:8317–8328. https://doi.org/10.1523/JNEUROSCI.0806-16.2016
Ziegler AN, Feng Q, Chidambaram S, Testai JM, Kumari E, Rothbard DE et al (2019) Insulin-like growth factor II: an essential adult stem cell niche constituent in brain and intestine. Stem Cell Reports 12:816–830. https://doi.org/10.1016/j.stemcr.2019.02.011
Zuccato C, Valenza M, Cattaneo E (2010) Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev 90:905–981. https://doi.org/10.1152/physrev.00041.2009
Zuleta A, Vidal RL, Armentano D, Parsons G, Hetz C (2012) AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington's disease. Biochem Biophys Res Commun 420:558–563. https://doi.org/10.1016/j.bbrc.2012.03.033
Acknowledgements
We are grateful to Dr. Oliver Bracko for providing IGF2 overexpression constructs. We thank the Harvard Brain Tissue Resource Center for providing HD post-mortem samples. We also thank Drs. Dimitri Krainc and Katarina Trajkovic for feedback on protocols to detect mHtt secretion. We also thank Dr. Cristina Alberini for feedback and providing tools to study IGF2 signaling. We thank Marioly Müller and Dr. Josefina Barrera and her team for their kind help taking the blood samples. We thank Dr. Felipe Oyarzun and Rodrigo Sierpe for the kindly help with the use of the Nanosight NS300. We specially acknowledge Carolina Jerez, Claudia Rivera, Valentina Castillo and Constanza Gonzalez for their technical assistance.
Funding
This work was directly funded by ANID/FONDAP program 15150012 (C.H. and R.L.V.), Millennium Institute P09-015-F (C.H. and R.L.V.), CONICYT-Brazil 441921/2016–7, FONDEF ID16I10223, FONDEF D11E1007 and FONDECYT 1180186 (C.H.). We also thank FONDECYT 3150097 (P.G-H.), FONDECYT 1191003 (R.L.V.), FONDECYT 1150069 (H.G.R.), CONICYT Ph.D. fellowship 21160843 (P.T-E.), CSC and the Postgraduate Student Research and Innovation Project of Jiangsu Province KYLX15_0558 (D.W.), the Hersenstichting and NWO-ALW (S.B.), NIH R01 NS100529 (L.M.E.) and NIH NS092829 (R.L.W.). ISF Legacy Heritage Fund (2394/17) (G.Z.L.)
Author information
Authors and Affiliations
Contributions
PG-H, PT-E, CH and RLV designed the study; PG-H and PT-E designed, performed and analyzed most of the in vitro and in vivo experiments; DW and SB designed, performed and analyzed the pulse-chase experiments; CS and FC analyzed the NTA experiments and assisted with the extracellular vesicle experiments; PC-C provided the information and blood samples from Chilean HD patients; DRH and CG-B designed and performed the experiments of actin remodeling; PT, KAL and BJG performed the microarray analysis using the brains samples from the XBP1cko mice; LP and RLW performed the quantitative proteomics in Neuro2a cells; CS and HGR performed the Ingenuity pathway analysis; SPS produced the AAV2-IGF2 and AAV2-Mock; GZL, NS and MS performed the experiments in HEK293 cells using the full-lenghtHttQ103-myc plasmid; SN and LME designed, performed and analyzed the experiments using MSNs derived from HD patients. AT designed, performed and analyzed the experiments using neurons derived from SCA3 patients.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this study. AAV-IGF2 for gene therapy and its use in misfolded protein diseases as Huntington’s disease (Chile no. 201603282 and PCT. provisional application no. PCT/CL2017/000040).
Data and materials availability
MTA may be required for access to AAV-IGF2 and some vectors. All data are available upon request from C.H.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 1209 kb) Supplementary video 1. Neuro2a treated with mock medium
Supplementary file2 (AVI 1561 kb) Supplementary video 2. Neuro2a treated with IGF2-enriched medium
401_2020_2183_MOESM3_ESM.tif
Supplementary file3 (TIF 1831 kb) Supplementary fig. 1 XBP1 deficiency does not alter Igf1 mRNA levels. a Igf2 mRNA levels were measured by real-time PCR in cDNA generated from striatal tissue of 9-month-old XBP1cKO-YAC128 mice. Data represent the average and SEM of the analysis of three animals per group. b Igf1 mRNA levels were measured by real-time PCR in cDNA generated from striatal tissue of 9-month-old XBP1cKO-YAC128 mice. Data represent the average and SEM of five animals. Statistically significant differences detected by one-tailed unpaired t-test (**: p < 0.01)
401_2020_2183_MOESM4_ESM.tif
Supplementary file4 (TIF 2666 kb) Supplementary fig. 2 IGF2 expression reduces polyQ79 levels. a PolyQ79-EGFP monomers from Fig. 2B were analyzed in whole cell extracts by western blot analysis using an anti-GFP antibody (left panel). PolyQ79-EGFP monomer levels after 24 and 48 h of expression were quantified and normalized to Hsp90 levels (middle and right panel, respectively) b Neuro2a cells were co-transfected with a polyQ79-EGFP expression vector and IGF2 plasmid or empty vector (Mock). 24 h later, cells were collected in Trizol and Gfp mRNA levels were measured by semi-quantitative PCR. Values represent the mean and SEM of at least three independent experiments. c Quantification of filter trap experiment from Fig. 2c (time point 48 h)
401_2020_2183_MOESM5_ESM.tif
Supplementary file5 (TIF 7168 kb) Supplementary fig. 3 IGF2 does not inhibit global protein synthesis or degradation. a HEK293T cells were co-transfected with GFP-mHttQ43 expression vector with IGF2 plasmid (right, upper panel) or empty vector (left, upper panel) and then pulse labeled with 35S for indicated time points. Autoradiography (AR) represents total proteins that were synthesized in the time of harvesting cells. All data were normalized to time point 0 h (lower panel). b HEK293T cells were co-transfected with GFP-mHttQ43 expression vector with IGF2 plasmid (right, upper panel) or empty vector (Mock) (left, upper panel) and then pulse labeled with 35S for indicated time points to follow the decay of the labeled protein while chasing with unlabeled precursor. Autoradiography (AR) represents total extracts in each time (upper panel). All data were normalized to time point 1 h (lower panel). c HEK293T cells were transfected with GFP-mHttQ43 and IGF2 expression vectors. Pulse was performed 24 h after transfection. Cells were treated with 30 μM chloroquine (CQ) or 1 µM bortezomib (Bort) at the beginning of the chasing for additional 21 h (upper panel). Ubiquitin, p62 and LC3 were measured as control of the autophagy inhibition
401_2020_2183_MOESM6_ESM.tif
Supplementary file6 (TIF 16029 kb) Supplementary fig. 4 Proteasome, autophagy or conventional secretion are not involved in the reduction in polyQ79 levels after IGF2 expression (control experiments). a Neuro2a cells were co-transfected with expression vectors for polyQ79-EGFP and IGF2 or empty vector (Mock). After 8 h, cells were treated with 1 μM MG132 for additional 16 h. PolyQ79-EGFP inclusions were visualized by fluorescence microscopy (upper panel) and polyQ79-EGFP aggregation levels measured by filter trap (lower panel). Image cropped from the same membrane and film. b Filter trap assay was performed to detect polyQ79-EGFP aggregates using the same cells extracts of Fig. 4e using an anti-GFP antibody (upper panel) and quantified (lower panel). Image cropped from the same membrane and film. c Neuro2a cells were transfected with IGF2 for 8 h and then treated with 1 μM lactacystin (Lact) or 1 μM MG132 for additional 16 h. Ubiquitin accumulation was measured by western blot to confirm the activity of the inhibitors. Hsp90 expression was measured as loading control. d Neuro2a cells where co-transfected with IGF2 plasmid or empty vector (Mock) in the presence or absence of polyQ79-EGFP expression vector for 8 h, and then treated with 30 μM chloroquine (CQ) for additional 16 h. p62 levels were monitored by western blot using anti-p62 antibody. Hsp90 expression was monitored as loading control (upper panel). p62 levels were quantified and normalized to Hsp90 (lower panel). e Neuro2a cells were co-transfected with polyQ79-EGFP and IGF2 expression vectors or empty vector (Mock) for 8 h and then treated with 30 μM CQ for additional 16 h. p62 and LC3 levels were measured by western blot as control for autophagy inhibitors in experiment shown in Fig. 4h. Hsp90 levels were determined as loading control. f HEK293T cells were co-transfected with polyQ79-EGFP and IGF2 expression vectors or empty vector (Mock). After 8 h, cells were treated with 30 μM chloroquine (CQ) for additional 16 h. PolyQ79-EGFP aggregation was analyzed in whole cells extracts by western blot using anti-GFP antibody. Hsp90 expression was monitored as loading control (left panel). PolyQ79-EGFP HMW species levels were quantified and normalized to Hsp90 levels (right panel). g Filter trap assay was performed using the same cells extracts analyzed in (f), and polyQ79-EGFP HMW species were detected using anti-GFP antibody (upper panel) and quantified (lower panel). h HEK293T cells were transiently transfected with IGF2 expressing vector (IGF2) or empty plasmid (Mock) for 8 h and then treated with 30 μM CQ for additional 16 h. p62 and LC3 levels were measured by western blot as control for autophagy inhibitors in experiment shown in Fig. 4f and Fig. 4g. Hsp90 levels were determined as loading control. i, j Neuro2a cells were co-transfected with polyQ79-EGFP and IGF2 or empty vector (Mock) for 8 h and then treated with 200 nM bafilomycin A1 (Baf) or 250 mM 3-methyladenine (3-MA) for additional 16 h. PolyQ79-EGFP HMW species were analyzed in cell lysates using western blot with an anti-GFP antibody. Hsp90 or GAPDH expressions were monitored as loading control. In all quantifications, average and SEM of at least three independent experiments are shown. Statistically significant differences detected by two-tailed unpaired t-test (**: p < 0.01; *: p < 0.05). k HEK293 cells were co-transfected with FL-Htt103Q and IGF2 plasmid or empty vector (Mock). 24 h later, culture media was collected and loaded for dot blot detection of FL-Htt103Q using anti-Htt antibody. l Neuro2a cells were co-transfected with a polyQ79-EGFP expression vector and IGF2 plasmid or empty vector (Mock). 48 h later, cell death was measured by FACS after propidium iodide staining. m NanoSight profile of exosomes purified form IGF2 expressing cells. Isolated exosomes from Neuro2a cells co-transfected with polyQ79-EGFP and IGF2 plasmid or empty vector (Mock) were analyzed by NanoSight nanotracking analysis and plotted by size to confirm proper purification method. n Western blot analysis of enriched fractions of microvesicles (MV) and exosomes using the markers Alix and CD63
401_2020_2183_MOESM7_ESM.tif
Supplementary file7 (TIF 3537 kb) Supplementary fig. 5 Gene therapy to deliver IGF2 into the brain. a Neuro2a cells were co-transfected with expression vectors for mHtt588Q95-RFP and IGF2 plasmid or empty vector (Mock) using an AAV back bound. After 24 h, mHttQ95-RFP inclusions were visualized by fluorescence microscopy (left panel). Protein aggregation was analyzed in whole cells extracts by western blot (right panel). Hsp90 was measured as loading control. b Igf2-HA expression was confirmed by conventional PCR in cDNA obtained from dissected striatum of animals co-injected with AAV mHtt588Q95-RFP and AAV-IGF2 or AAV-Mock showed in Fig. 8a. c Igf2-HA expression was confirmed by conventional PCR in cDNA obtained from dissected striatum of YAC128 animals injected with AAV-IGF2 or AAV-Mock intra-cerebroventricular at neonatal stage P1-P2 shown in Fig. 8c. d Disease progression was monitored once every two weeks, using the rotarod test. YAC128 and littermate control mice were injected with AAVs as indicated in Fig. 8c. Motor performance was monitored once every two weeks from 2-month to 8-month old. The analysis shows the average of the group at each time point
Rights and permissions
About this article
Cite this article
García-Huerta, P., Troncoso-Escudero, P., Wu, D. et al. Insulin-like growth factor 2 (IGF2) protects against Huntington’s disease through the extracellular disposal of protein aggregates. Acta Neuropathol 140, 737–764 (2020). https://doi.org/10.1007/s00401-020-02183-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-020-02183-1