Abstract
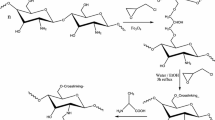
A novel cross-linked chitosan-poly(aspartic acid) chelating resin was synthesized by combining chitosan and poly(aspartic acid) derivative with crosslinker. The structure and morphology of the resin containing disulfide bonds were characterized by 1H NMR, FT-IR, Raman spectra, and SEM. Adsorption of Pb(II) and Hg(II) from aqueous solution by the resulted chelating resin was investigated in batch techniques. The result of the Raman spectra suggests that the disulfide bonds in the resin also play an important role in the adsorption of Pb(II) and Hg(II). The kinetic data were fitted to pseudo-first-order, pseudo-second-order, and intraparticle diffusion models, which is suggestive of following closely the pseudo-second-order kinetic model. Equilibrium data were fitted to Langmuir, Freundlich, and D–R isotherm models. The results of equilibrium isotherm reveal that the empirical Langmuir equation provides an accurate description of the experimental data under the studied concentration range. In addition, thermodynamic and regeneration properties of the adsorbent were also studied.














Similar content being viewed by others
References
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55:77–93. doi:10.1016/j.carbpol.2003.08.005
Bhatnagar A, Sillanpää M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interf 152:26–38. doi:10.1016/j.cis.2009.09.003
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. doi:10.1016/j.progpolymsci.2006.06.001
Wu F-C, Tseng R-L, Juang R-S (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manag 91:798–806. doi:10.1016/j.jenvman.2009.10.018
Zhao Y, Tan TW (2006) Poly(aspartic acid) super-absorbent resin produced by chemical crosslinking and physical freeze/thawing. Macromol Chem Phys 207:1297–1305. doi:10.1002/macp.200600168
Sun B, Mi ZT, Jie OY, An G, Song XK (2005) Pb2+ binding by polyaspartyl polymers and their application to Pb2+ removal from glycyrrhizin. J Appl Polym Sci 97:2215–2220. doi:10.1002/app.21867
Anbergen U, Opperman W (1990) Elasticity and swelling behaviour of chemically crosslinked cellulose ethers in aqueous systems. Polymer 31:1854–1858. doi:10.1016/0032-3861(90)90006-K
Wan Ngah WS, Fatinathan S (2010) Pb(II) biosorption using chitosan and chitosan derivatives beads: equilibrium, ion exchange and mechanism studies. J Environ Sci 22:338–346. doi:10.1016/S1001-0742(09)60113-3
Sun B, Mi ZT, An G, Liu GZ, Zou JJ (2009) Preparation of biomimetic materials made from polyaspartyl polymer and chitosan for heavy-metal removal. Ind Eng Chem Res 48:9823–9829. doi:10.1021/ie900673h
Harish Prashanth KV, Tharanathan RN (2007) Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Sci Technol 18:117–131. doi:10.1016/j.tifs.2006.10.022
Behpour M, Ghoreishi SM, Mohammadi N, Soltani N, Salavati-Niasari M (2010) Investigation of some Schiff base compounds containing disulfide bond as HCl corrosion inhibitors for mild steel. Corros Sci 52:4046–4057. doi:10.1016/j.corsci.2010.08.020
Morad MS, Kamal El-Dean AM (2006) 2,2'-Dithiobis(3-cyano-4,6-dimethylpyridine): a new class of acid corrosion inhibitors for mild steel. Corros Sci 48:3398–3412. doi:10.1016/j.corsci.2005.12.006
Chen S, Yue Q, Gao B, Li Q, Xu X (2011) Removal of Cr(VI) from aqueous solution using modified corn stalks: characteristic, equilibrium, kinetic and thermodynamic study. Chem Eng J 168:909–917. doi:10.1016/j.cej.2011.01.063
Miretzky P, Cirelli AF (2009) Hg(II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167:10–23. doi:10.1016/j.jhazmat.2009.01.060
Cao H, Ma X, Sun S, Su H, Tan T (2010) A new photocrosslinkable hydrogel based on a derivative of polyaspartic acid for the controlled release of ketoprofen. Polym Bull 64:623–632. doi:10.1007/s00289-009-0215-z
Repo E, Warchol JK, Kurniawan TA, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161:73–82. doi:10.1016/j.cej.2010.04.030
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645. doi:10.1016/j.jhazmat.2008.06.042
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–346. doi:10.1016/j.jhazmat.2007.10.031
Zhou L, Wang Y, Liu Z, Huang Q (2009) Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J Hazard Mater 161:995–1002. doi:10.1016/j.jhazmat.2008.04.078
Tarley CRT, Andrade FN, Santana H, Zaia DAM, Beijo LA, Segatelli MG (2012) Ion-imprinted polyvinylimidazole-silica hybrid copolymer for selective extraction of Pb(II): characterization and metal adsorption kinetic and thermodynamic studies. React Funct Polym 72:83–91. doi:10.1016/j.reactfunctpolym.2011.10.008
Bhattacharyya KG, Gupta SS (2007) Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: influence of acid activation. J Colloid Interf Sci 310:411–424. doi:10.1016/j.jcis.2007.01.080
Chen CY, Chiang CL, Chen CR (2007) Removal of heavy metal ions by a chelating resin containing glycine as chelating groups. Sep Purif Technol 54:396–403. doi:10.1016/j.seppur.2006.10.020
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J Hazard Mater 177:962–970. doi:10.1016/j.jhazmat.2010.01.012
Li ZT, Guo J, Zhang JS, Zhao YP, Lv L, Ding C, Zhang XZ (2010) Chitosan-graft-polyethylenimine with improved properties as a potential gene vector. Carbohydr Polym 80:254–259. doi:10.1016/j.carbpol.2009.11.021
Guinesi LS, Cavalheiro ÉTG (2006) Influence of some reactional parameters on the substitution degree of biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Carbohydr Polym 65:557–561. doi:10.1016/j.carbpol.2006.01.030
Xiao Y, Zhou XH (2008) Synthesis and properties of a novel cross-linked chitosan resin modified by l-lysine. React Funct Polym 68:1281–1289. doi:10.1016/j.reactfunctpolym.2008.06.015
Wang S, Yu D (2010) Adsorption of Cd(II), Pb(II), and Ag(I) in aqueous solution on hollow chitosan microspheres. J Appl Polym Sci 118:733–739. doi:10.1002/app.32496
Monier M (2012) Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan–thioglyceraldehyde Schiff’s base. Int J Biol Macromol 50:773–781. doi:10.1016/j.ijbiomac.2011.11.026
Shafaei A, Ashtiani FZ, Kaghazchi T (2007) Equilibrium studies of the sorption of Hg(II) ions onto chitosan. Chem Eng J 133:311–316. doi:10.1016/j.cej.2007.02.016
Liu X, Hu Q, Fang Z, Zhang X, Zhang B (2009) Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25:3–8. doi:10.1021/la802754t
Wang L, Xing R, Liu S, Cai S, Yu H, Feng J, Li R, Li P (2010) Synthesis and evaluation of a thiourea-modified chitosan derivative applied for adsorption of Hg(II) from synthetic wastewater. Int J Biol Macromol 46:524–528. doi:10.1016/j.ijbiomac.2010.03.003
Vieira RS, Guibal E, Silva EA, Beppu MM (2007) Adsorption and desorption of binary mixtures of copper and mercury ions on natural and crosslinked chitosan membranes. Adsorption 13:603–611. doi:10.1007/s10450-007-9050-4
Gohari M, Hosseini SN, Sharifnia S, Khatami M (2013) Enhancement of metal ion adsorption capacity of Saccharomyces cerevisiae’s cells by using disruption method. J Taiwan Inst Chem Eng. doi:10.1016/j.jtice.2013.01.002
Ma F, Qu R, Sun C, Wang C, Ji C, Zhang Y, Yin P (2009) Adsorption behaviors of Hg(II) on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers. J Hazard Mater 172:792–801. doi:10.1016/j.jhazmat.2009.07.066
Niu L, Deng SB, Yu G, Huang J (2010) Efficient removal of Cu(II), Pb(II), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization. Chem Eng J 165:751–757. doi:10.1016/j.cej.2010.08.053
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 50:1095–1105
Wan Ngah WS, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91:958–969. doi:10.1016/j.jenvman.2009.12.003
Chen AH, Liu SC, Chen CY, Chen CY (2008) Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the cross-linked chitosan with epichlorohydrin. J Hazard Mater 154:184–191. doi:10.1016/j.jhazmat.2007.10.009
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interf Sci 276:47–52. doi:10.1016/j.jcis.2004.03.048
Aksu Z, Tatlı Aİ, Tunç Ö (2008) A comparative adsorption/biosorption study of Acid Blue 161: effect of temperature on equilibrium and kinetic parameters. Chem Eng J 142:23–39. doi:10.1016/j.cej.2007.11.005
Li XL, Yang LQ, Li YF, Ye ZF, He AX (2012) Efficient removal of Cd2+ from aqueous solutions by adsorption on PS-EDTA resins: equilibrium, isotherms and kinetic studies. J Environ Eng-ASCE 138:940–948. doi:10.1061/(ASCE)EE1943-7870.0000553
Choy KKH, Porter JF, McKay G (2004) Intraparticle diffusion in single and multicomponent acid dye adsorption from wastewater onto carbon. Chem Eng J 103:133–145. doi:10.1016/j.cej.2004.05.012
Özacar M, Şengil İA, Türkmenler H (2008) Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin. Chem Eng J 143:32–42. doi:10.1016/j.cej.2007.12.005
Krishnapriya KR, Kandaswamy M (2010) A new chitosan biopolymer derivative as metal-complexing agent: synthesis, characterization, and metal(II) ion adsorption studies. Carbohydr Res 345:2013–2022. doi:10.1016/j.carres.2010.06.005
Krishnapriya KR, Kandaswamy M (2009) Synthesis and characterization of a crosslinked chitosan derivative with a complexing agent and its adsorption studies toward metal(II) ions. Carbohydr Res 344:1632–1638. doi:10.1016/j.carres.2009.05.025
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Almeida VC, Tambourgi EB (2011) Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 275:187–196. doi:10.1016/j.desal.2011.02.056
Repo E, Warchoł JK, Bhatnagar A, Sillanpää M (2011) Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J Colloid Interf Sci 358:261–267. doi:10.1016/j.jcis.2011.02.059
Yan WL, Bai R (2005) Adsorption of lead and humic acid on chitosan hydrogel beads. Water Res 39:688–698. doi:10.1016/j.watres.2004.11.007
Wan M-W, Kan C-C, Rogel BD, Dalida MLP (2010) Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr Polym 80:891–899. doi:10.1016/j.carbpol.2009.12.048
Futalan CM, Kan C-C, Dalida ML, Hsien K-J, Pascua C, Wan M-W (2011) Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr Polym 83:528–536. doi:10.1016/j.carbpol.2010.08.013
Laus R, Costa TG, Szpoganicz B, Fávere VT (2010) Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J Hazard Mater 183:233–241. doi:10.1016/j.jhazmat.2010.07.016
Zhu Y, Hu J, Wang J (2012) Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J Hazard Mater 221–222:155–161. doi:10.1016/j.jhazmat.2012.04.026
Fan L, Luo C, Sun M, Li X, Qiu H (2013) Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloid Surf B 103:523–529. doi:10.1016/j.colsurfb.2012.11.006
Chauhan D, Sankararamakrishnan N (2008) Highly enhanced adsorption for decontamination of lead ions from battery wastewaters using chitosan functionalized with xanthate. Bioresour Technol 99:9021–9024. doi:10.1016/j.biortech.2008.04.024
He YQ, Zhang NN, Wang XD (2011) Adsorption of graphene oxide/chitosan porous materials for metal ions. Chin Chem Lett 22:859–862. doi:10.1016/j.cclet.2010.12.049
Li X, Li Y, Ye Z (2011) Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem Eng J 178:60–68. doi:10.1016/j.cej.2011.10.012
Dongre R, Thakur M, Ghugal D, Meshram J (2012) Bromine pretreated chitosan for adsorption of lead (II) from water. B Mater Sci 35:875–884. doi:10.1007/s12034-012-0359-6
Wang R-M, Xie X, Wang J-Q, Pan S-J, Wang Y-P, Xia C-G (2004) Preparation and adsorption properties of modified chitosan. Polym Adv Technol 15:52–54. doi:10.1002/pat.450
Tang X, Zhang X, Guo C, Zhou A (2007) Adsorption of Pb2+ on chitosan cross-linked with triethylene-tetramine. Chem Eng Technol 30:955–961. doi:10.1002/ceat.200600379
Ge H, Fan X (2011) Adsorption of Pb2+ and Cd2+ onto a novel activated carbon-chitosan complex. Chem Eng Technol 34:1745–1752. doi:10.1002/ceat.201000182
Saiano F, Ciofalo M, Cacciola SO, Ramirez S (2005) Metal ion adsorption by Phomopsis sp. biomaterial in laboratory experiments and real wastewater treatments. Water Res 39:2273–2280. doi:10.1016/j.watres.2005.04.022
Yang Z, Shu J, Zhang L, Wang Y (2006) Preparation and adsorption behavior for metal ions of cyclic polyamine derivative of chitosan. J Appl Polym Sci 100:3018–3023. doi:10.1002/app.23690
Paulino AT, Santos LB, Nozaki J (2008) Removal of Pb2+, Cu2+, and Fe3+ from battery manufacture wastewater by chitosan produced from silkworm chrysalides as a low-cost adsorbent. React Funct Polym 68:634–642. doi:10.1016/j.reactfunctpolym.2007.10.028
Ge H, Huang S (2010) Microwave preparation and adsorption properties of EDTA-modified cross-linked chitosan. J Appl Polym Sci 115:514–519. doi:10.1002/app.30843
Zhang G, Qu R, Sun C, Ji C, Chen H, Wang C, Niu Y (2008) Adsorption for metal ions of chitosan coated cotton fiber. J Appl Polym Sci 110:2321–2327. doi:10.1002/app.27515
Sun X, Peng B, Ji Y, Chen J, Li D (2009) Chitosan(Chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J 55:2062–2069. doi:10.1002/aic.11797
Tran HV, Tran LD, Nguyen TN (2010) Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater Sci Eng C 30:304–310. doi:10.1016/j.msec.2009.11.008
Paulino AT, Guilherme MR, Reis AV, Tambourgi EB, Nozaki J, Muniz EC (2007) Capacity of adsorption of Pb2+ and Ni2+ from aqueous solutions by chitosan produced from silkworm chrysalides in different degrees of deacetylation. J Hazard Mater 147:139–147. doi:10.1016/j.jhazmat.2006.12.059
Akkaya R, Ulusoy U (2008) Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO2 2+, and Th4+. J Hazard Mater 151:380–388. doi:10.1016/j.jhazmat.2007.05.084
Zhou L, Liu Z, Liu J, Huang Q (2010) Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination 258:41–47. doi:10.1016/j.desal.2010.03.051
Jeon C, Park KH (2005) Adsorption and desorption characteristics of mercury(II) ions using aminated chitosan bead. Water Res 39:3938–3944. doi:10.1016/j.watres.2005.07.020
Vieira RS, Beppu MM (2006) Dynamic and static adsorption and desorption of Hg(II) ions on chitosan membranes and spheres. Water Res 40:1726–1734. doi:10.1016/j.watres.2006.02.027
Monier M, Abdel-Latif DA (2012) Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J Hazard Mater 209–210:240–249. doi:10.1016/j.jhazmat.2012.01.015
Qu R, Sun C, Ma F, Zhang Y, Ji C, Xu Q, Wang C, Chen H (2009) Removal and recovery of Hg(II) from aqueous solution using chitosan-coated cotton fibers. J Hazard Mater 167:717–727. doi:10.1016/j.jhazmat.2009.01.043
Atia AA (2005) Studies on the interaction of mercury(II) and uranyl(II) with modified chitosan resins. Hydrometallurgy 80:13–22. doi:10.1016/j.hydromet.2005.03.009
Jeon C, Holl WH (2003) Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res 37:4770–4780. doi:10.1016/S0043-1354(03)00431-7
Donia AM, Atia AA, Elwakeel KZ (2008) Selective separation of mercury(II) using magnetic chitosan resin modified with Schiff’s base derived from thiourea and glutaraldehyde. J Hazard Mater 151:372–379. doi:10.1016/j.jhazmat.2007.05.083
Vieira RS, Beppu MM (2006) Interaction of natural and crosslinked chitosan membranes with Hg(II) ions. Colloid Surf A 279:196–207. doi:10.1016/j.colsurfa.2006.01.026
Lasheen MR, Ammar NS, Ibrahim HS (2012) Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: equilibrium and kinetic studies. Solid State Sci 14:202–210. doi:10.1016/j.solidstatesciences.2011.11.029
Acknowledgments
We would like to appreciate for the financial supports from the Central Universities Foundation of Harbin Engineering University, China (HEUCFT1009, HEUCF20130910004, HEUCF201403008) and the National Science Foundation of China (21204014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X.J., Cai, J.C., Zhang, Z.H. et al. Investigation of removal of Pb(II) and Hg(II) by a novel cross-linked chitosan-poly(aspartic acid) chelating resin containing disulfide bond. Colloid Polym Sci 292, 2157–2172 (2014). https://doi.org/10.1007/s00396-014-3240-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3240-x