Abstract
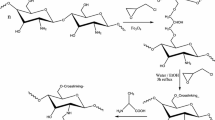
Chitosan (Chi) beads were conjugated with three different amino acids [namely, glutamic acid (GLU), methionine (MET), and taurine (TAU)] aiming to increase the divalent copper ions uptake in aqueous media. Scanning Electron Microscopy evidenced the development of a large porous structure after amino acid functionalization, associated with the increase in a number of amino groups in the polymer backbone. X-Ray Photoelectron Spectroscopy and Fourier-Transform Infrared Spectra analyses were also employed to assess the conjugation of these three different amino acids in chitosan backbone. Adsorption experiments were conducted in a batch process, at 298 K, and kinetic data indicated a slightly better fitting for the pseudo-first-order model when compared to pseudo-second order. Intraparticle diffusion model suggested a three-step mechanism for Cu(II) adsorption kinetics, limited by the third step, the intraparticle diffusion. The isotherm data fitting to the traditional Langmuir and Freundlich models indicated a better fit for the former case. The amino acid conjugation resulted in the increase of the maximum adsorption capacity for Cu(II) from 1.30 mmol g−1 prior to amino acid conjugation to values as high as 2.31 mmol g−1, 2.40 mmol g−1 and 2.68 mmol g−1 for Chi–TAU, Chi–GLU, and Chi–MET, respectively. These results are attributed to the introduction of additional amino groups and new carboxylate and amino acid residues into the chitosan backbone, which might also be explored for amino acid demanding applications.








Similar content being viewed by others
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Li J, Tong J, Li X, Yang Z, Zhang Y, Diao G (2016) Facile microfluidic synthesis of copolymer hydrogel beads for the removal of heavy metal ions. J Mater Sci 51(23):10375–10385
USEPA (United Station Environmental Protection Agency) (2010) Lead and copper rule monitoring and reporting guidance for public water systems (EPA-816-R-02-009). Ground Water and Drinking Water Division, Water Programs, Washington, DC
Chen Q, Luo Z, Hills C, Xue G, Tyrer M (2009) Precipitation of heavy metals from wastewater using simulated flue gas: sequent additions of fly ash, lime and carbon dioxide. Water Res 43(10):2605–2614
Samper E, Rodríguez M, De la Rubia M, Prats D (2009) Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep Purif Technol 65(3):337–342
Khelifa A, Moulay S, Naceur A (2005) Treatment of metal finishing effluents by the electroflotation technique. Desalination 181(1–3):27–33
Chang Q, Wang G (2007) Study on the macromolecular coagulant PEX which traps heavy metals. Chem Eng Sci 62(17):4636–4643
Rabelo R, Vieira R, Luna F, Guibal E, Beppu M (2012) Adsorption of copper (II) and mercury (II) ions onto chemically-modified chitosan membranes: equilibrium and kinetic properties. Adsorpt Sci Technol 30(1):1–21
Yu Y, Peng R, Yang C, Tang Y (2015) Eco-friendly and cost-effective superabsorbent sodium polyacrylate composites for environmental remediation. J Mater Sci 50(17):5799–5808
Albarelli JQ, Rabelo RB, Santos DT, Beppu MM, Meireles MAA (2011) Effects of supercritical carbon dioxide on waste banana peels for heavy metal removal. J Supercrit Fluids 58(3):343–351
Oliveira LS, Franca AS, Alves TM, Rocha SD (2008) Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J Hazard Mater 155(3):507–512
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632
Deans JR, Dixon BG (1992) Uptake of Pb2 + and Cu2 + by novel biopolymers. Water Res 26(4):469–472
Vieira RS, Lisa M, Oliveira M, Guibal E, Rodríguez-castellón E, Beppu MM (2011) Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: an XPS investigation of mechanism. Colloids Surf A 374(1–3):108–114
Steenkamp G, Keizer K, Neomagus H, Krieg H (2002) Copper (II) removal from polluted water with alumina/chitosan composite membranes. J Membr Sci 197(1):147–156
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38(1):43–74
Beppu MM, Vieira RS, Aimoli CG, Santana CC (2007) Crosslinking of chitosan membranes using glutaraldehyde: effect on ion permeability and water absorption. J Membr Sci 301(1–2):126–130
Fu H, Kobayashi T (2010) Self-assembly functionalized membranes with chitosan microsphere/polyacrylic acid layers and its application for metal ion removal. J Mater Sci 45(24):6694–6700
Boggione MaJ, Mahl CRA, Beppu MM, Farruggia B (2017) Synthesis and characterization of chitosan membranes functionalized with amino acids and copper for adsorption of endoglucanase. Powder Technol 315:250–257
Demir AG, Taketa TB, Tolouei R, Furlan V, Paternoster C, Beppu MM, Mantovani D, Previtali B (2015) Laser surface structuring affects polymer deposition, coating homogeneity, and degradation rate of Mg alloys. Mater Lett 160:359–362
Vasconcellos FC, Goulart GAS, Beppu MM (2011) Production and characterization of chitosan microparticles containing papain for controlled release applications. Powder Technol 205(1–3):65–70
Vieira RS, Beppu MM (2005) Mercury ion recovery using natural and crosslinked chitosan membranes. Adsorption 11:731–736
Oshita K, Sabarudin A, Takayanagi T, Oshima M, Motomizu S (2009) Adsorption behavior of uranium (VI) and other ionic species on cross-linked chitosan resins modified with chelating moieties. Talanta 79(4):1031–1035
Inoue K, Yoshizuka K, Ohto K (1999) Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal Chim Acta 388(1):209–218
Casettari L, Vllasaliu D, Lam JKW, Soliman M, Illum L (2012) Biomedical applications of amino acid-modi fi ed chitosans: a review. Biomaterials 33(30):7565–7583
Sano T, Murase I (1980) US Patent 4,200,735. U.S. Patent and Trademark Office, Washington, DC
Adhikari CR, Parajuli D, Inoue K, Ohto K, Kawakita H (2008) Pre-concentration and separation of heavy metal ions by chemically modified waste paper gel. Chemosphere 72(2):182–188
Li N, Bai R (2005) Copper adsorption on chitosan-cellulose hydrogel beads: behaviors and mechanisms. Sep Purif Technol 42(3):237–247
Ngah WSW, Fatinathan S (2008) Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem Eng J 143(1–3):62–72
Zhao F, Yu B, Yue Z, Wang T, Wen X, Liu Z, Zhao C (2007) Preparation of porous chitosan gel beads for copper(II) ion adsorption. J Hazard Mater 147(1–2):67–73
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5(2):212–223
Al-Degs YS, El-Barghouthi MI, Issa AA, Khraisheh MA, Walker GM (2006) Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Res 40(14):2645–2658
Monteiro OA, Airoldi C (1999) Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int J Biol Macromol 26(2):119–128
Kurita K, Koyama Y, Taniguchi A (1986) Studies on chitin. IX. Crosslinking of water-soluble chitin and evaluation of the products as adsorbents for cupric ion. J Appl Polym Sci 31(5):1169–1176
Wan Ngah WS, Endud CS, Mayanar R (2002) Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym 50(2):181–190
Wan Ngah WS, Kamari A, Koay YJ (2004) Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int J Biol Macromol 34(3):155–161
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456
Ho Y-S, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34(3):735–742
Lagergren SY (1898) Zur Theorie der sogenannten adsorption gelöster stoffe. Handlingar, Stockholm
Baroni P, Vieira RS, Meneghetti E, da Silva MGC, Beppu MM (2008) Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J Hazard Mater 152(3):1155–1163
Papageorgiou SK, Kouvelos EP, Katsaros FK (2008) Calcium alginate beads from Laminaria digitata for the removal of Cu + 2 and Cd + 2 from dilute aqueous metal solutions. Desalination 224(1–3):293–306
Ho YS, Wase DAJ, Forster CF (1995) Batch nickel removal from aqueous solution by sphagnum moss peat. Water Res 29(5):1327–1332
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div 89:31–60
Lee ST, Mi FL, Shen YJ, Shyu SS (2001) Equilibrium and kinetic studies of copper(II) ion uptake by chitosan-tripolyphosphate chelating resin. Polymer 42(5):1879–1892
Findon A, McKay G, Blair HS (1993) Transport studies for the sorption of copper ions by chitosan. J Environ Sci Health A 28:173–185
Popuri SR, Vijaya Y, Boddu VM, Abburi K (2009) Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour Technol 100(1):194–199
Beppu M, Arruda E, Vieira R, Santos N (2004) Adsorption of Cu (II) on porous chitosan membranes functionalized with histidine. J Membr Sci 240(1):227–235
Piron E, Domard A (1997) Interaction between chitosan and uranyl ions. Part 1. Role of physicochemical parameters. Int J Biol Macromol 21(4):327–335
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta 396(1–2):153–166
Ritthidej GC, Phaechamud T, Koizumi T (2002) Moist heat treatment on physicochemical change of chitosan salt films. Int J Pharm 232(1–2):11–22
Mary MB, Umadevi M, Pandiarajan S, Ramakrishnan V (2004) Vibrational spectral studies of l-methionine l-methioninium perchlorate monohydrate. Spectrochim Acta A 60(11):2643–2651
Triebel S, Sproll C, Reusch H, Godelmann R, Lachenmeier DW (2007) Rapid analysis of taurine in energy drinks using amino acid analyzer and Fourier transform infrared (FTIR) spectroscopy as basis for toxicological evaluation. Amino Acids 33(3):451–457
Acknowledgements
The authors acknowledge the financial support from Brazilian governmental agencies Coordination for the Improvement of Higher Education Personnel (CAPES: Procad 88882.151600/2017-01 and PNPD/FEQ), National Council for Scientific and Technological Development (CNPq) and São Paulo Research Foundation (FAPESP) (Grants Nos. 2013/05135-1 and 2016/10193-9). We also thank the Massachusetts Institute of Technology (MIT) Center for Materials Science and Engineering (CMSE), and Elisabeth Shaw for supporting the XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahl, C.R.A., Taketa, T.B., Bataglioli, R.A. et al. Chitosan Functionalization with Amino Acids Yields to Higher Copper Ions Adsorption Capacity. J Polym Environ 26, 4338–4349 (2018). https://doi.org/10.1007/s10924-018-1306-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1306-4